Contact Section Leader
Prof.Dr.med.Dr.phil. Eva Winkler
E-mail: eva.winkler@med.uni-heidelberg.de
Due to the impressing medical & technological progress fostered by structural and systemic changes in health care, ethical issues in oncology and research constantly gain importance. Such issues include, for instance, the informed consent process in research, patient’s rights and the right to self-determination or the ethical justification of expensive treatments facing limited resources in health care.
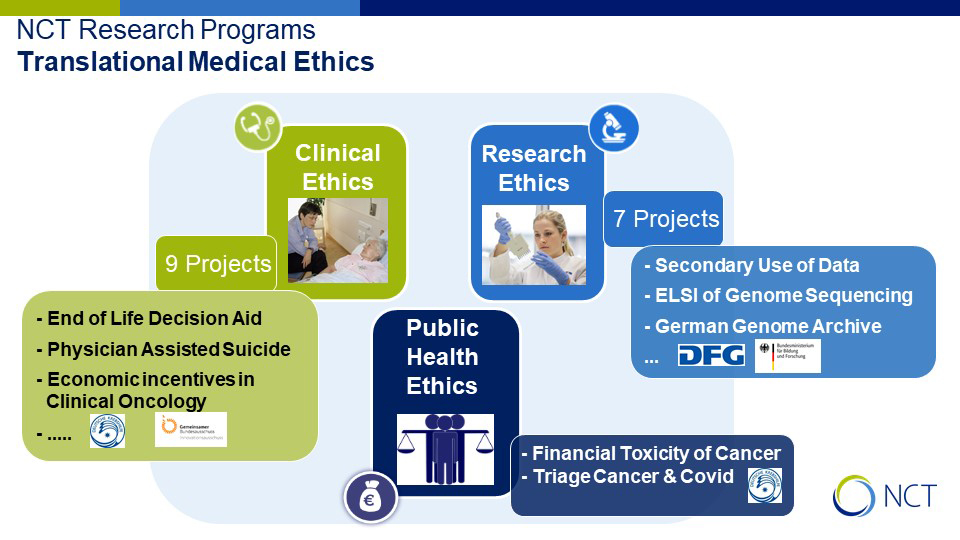
The concept of Empirical Ethics combines empirical studies in patient care and research with ethical analysis (cf. figure 1).