COGNITION (eBC) and CATCH (mBC)
Breast cancer: Diagnostic Platforms
- integrative biomarker-driven personalised therapy based on the individual genetic profile of the tumor
- in addition to conventional characteristics (e.g. histological markers), prognostic and predictive markers, the individual genomic tumor biology and biomarker profile supports therapy decisions
- depending on their tumor stage breast cancer patients can be allocated to two different diagnostic-platforms: COGNITION (early breast cancer) or CATCH (advanced-stage / metastatic breast cancer
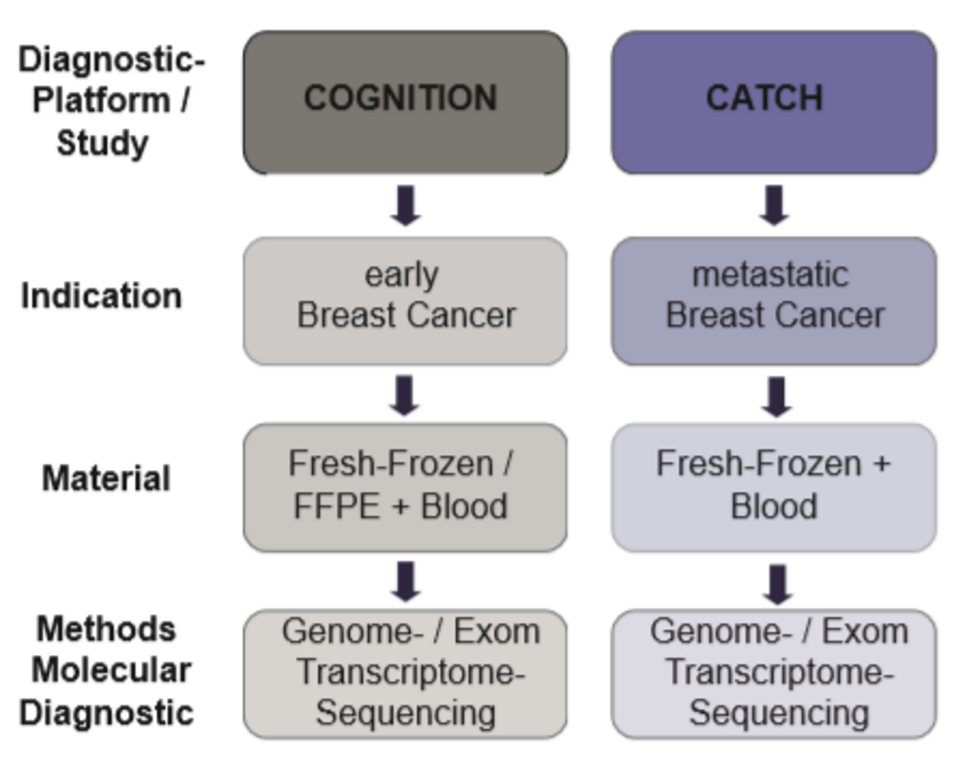
Overview of breast cancer-specific platforms
| COGNITION (eBC) | CATCH (mBC) | |
| Indication | Early breast cancer that can be treated with curative intention with indication for neoadjuvant therapy (regardless of subtype) and patients with poor response to neoadjuvant therapy | Metastatic breast cancer at the time of diagnosis or progression |
| Material requirement | Native material (fresh-frozen) a) baseline (before neoadjuvant therapy) | Native material (fresh-frozen): metastatic lesion (biopsy + surgical material) +blood |
| Method profiling (Illumina) |
|
|
COGNITION (early breast cancer)
Comprehensive assessment of clinical features, genomics and further molecular markers to identify patients with early breast cancer for enrolment on marker driven trials: early high-risk breast carcinoma.
Objective
COGNITION aims to offer a comprehensive molecular-genetic profiling of the therapy-resistant lesion after neoadjuvant therapy for early high-risk breast cancer patients who present with a residual bulk tumor (non-pCR or high CPS-EG score) after neoadjuvant therapy. This approach allows for an individualised, risk-adapted, biomarker-stratified therapy recommendation following standard post-neoadjuvant therapy in order to decrease the substantial risk of recurrence and metastasis.
Neoadjuvant chemotherapies (NACT) represent the cornerstone of treating Luminal B, HER2 positive and Triple Negative breast cancer. The pathological Complete Response (pCR) and the CPS-EG score serve as solid intermediate endpoints and surrogate markers for overall survival and enable identification of patients with a high risk of recurrence. Post-neoadjuvant trials such as the CREATE-X and the KATHERINE already underlined the potential of intensified postneoadjuvant chemotherapy to lower the risk for relapse. We hypothesize that high-risk patients benefit from an individualized therapy following surgery and standard-post-neoadjuvant therapy adjusted according the molecular profile of their invasive residual tumor to decrease the risk of recurrence and especially distant metastases.
Within COGNITION, precision oncology for high-risk breast cancer patients is implemented in daily clinical practice. Within the framework of the study, a comprehensive molecular diagnostics (predominantly high-throughput sequencing) is applied for high-risk patients to generate a comprehensive genotype and biomarker analysis of the individual tumor.
For this purpose, primary tumors prior to neoadjuvant therapy as well as the residual tumor after neoadjuvant therapy at the time of surgery are analysed by molecular profiling (especially whole-genome / whole-exome and transcriptome sequencing) for a differentiated adequate diagnosis to stratify the post-neoadjuvant treatment according to its genomic patterns. This strategy empowers therapy escalation (in addition to the standard) during the prospectively linked phase II interventional trial (COGNITION-GUIDE) with targeted substances. COGNITION-GUIDE is currently planned and will be available soon (see below) in order to access if precision oncology in the curative setting can lower the substantial risk of disseminated metastasis and hence to increase cure rates.
Inclusion criteria:
- Patients with histological confirmed early breast cancer who are treated with curative intention (Stage I-III) and who have an indication for neoadjuvant therapy (all subtypes)
- Patients who do not response to neoadjuvant therapy (inclusion prior to surgery to extract fresh tissue for the tumor genome sequencing is essential)
- Possibility for tissue (biopsy) and blood sampling or, in case of no response, FFPE surgery material with a content of tumor cells of >20 %
Exclusion criteria:
- metastatic breast cancer
- Inadequate organ function
- ECOG >1
- Impossibility of tissue / blood sampling
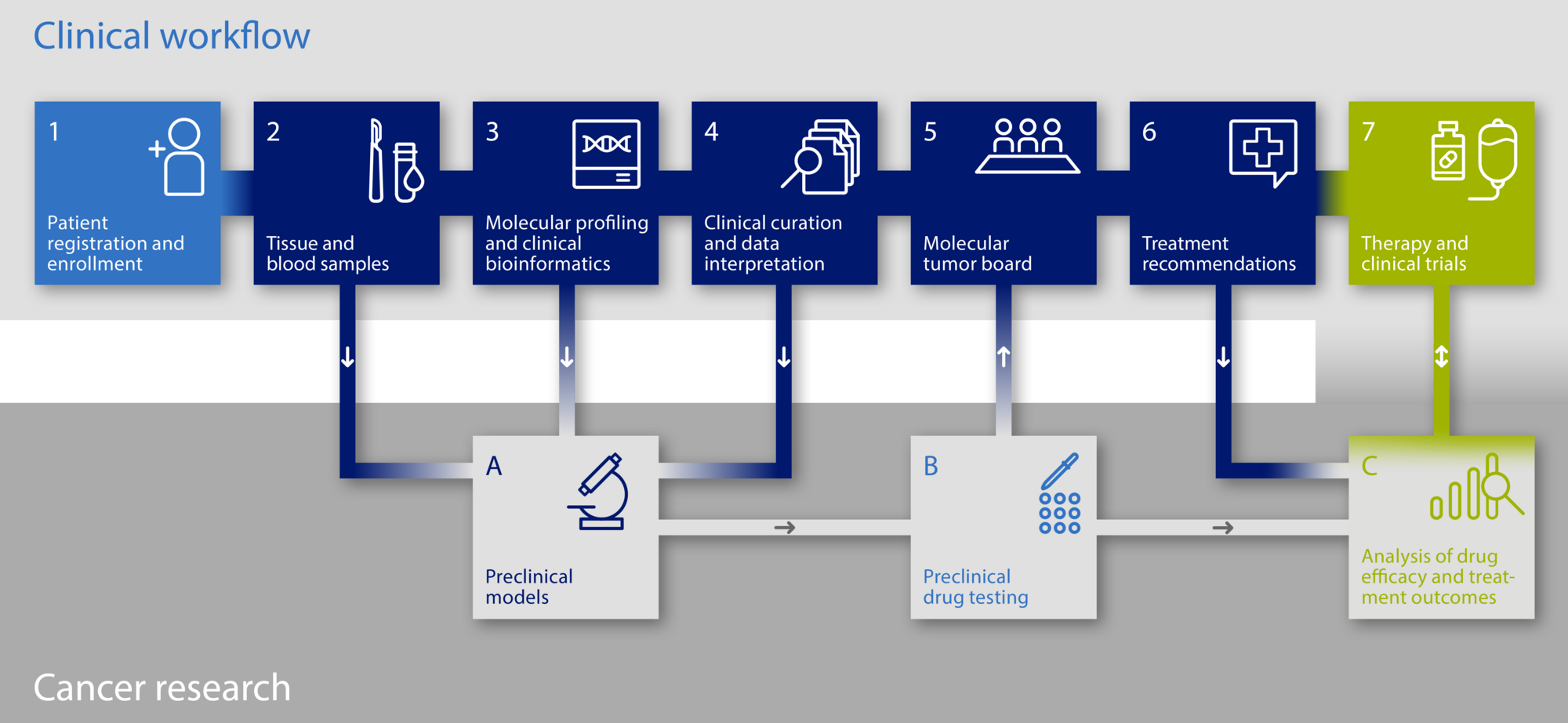
The diagnostic platforms follow a standardised workflow:
The diagnostic platforms follow a standardised workflow:
1. Information / enrolment at the NCT Heidelberg or any other participating study center
2. Acquisition of biomaterial (tissue + blood) for molecular analyses in the course of standard routine procedures or as a study specific measure
- Tumor tissue: Fresh tissue samples (no FFPE if possible) as pre-requesite for downstream molecular-genetic analyses
- Blood withdrawal (comparative sample of normal body cells to define tumor-specific genetic alterations)
3. Evaluation tumour cell content fresh tissue sample (minimum content 20 % tumor cells) and tumor infiltrating lymphocytes
4. Molecular Analyses: Tumor-Genome-Sequencing (DKFZ Heidelberg) / Next Generation Sequencing
- Analyte-Isolation (DNA, RNA, Protein, Sample Processing Lab, DKFZ)
- Sample preparation for high-throughput sequencing (Genomics Core Facility, DKFZ)
- Method: whole-genome/ whole-exome and transcriptome sequencing
5. Discussion of molecular-genetic findings within the interdisciplinary molecular tumor board
- Participants: Oncologists, bioinformaticians, molecular biologists, pathologists, human geneticists
- Prioritisation of therapy-relevant gene alterations and molecular targets
- Generating a therapy plan combined in a molecular report
6. Discussion of molecular-genetic findings and consideration of possible treatment options with the patient
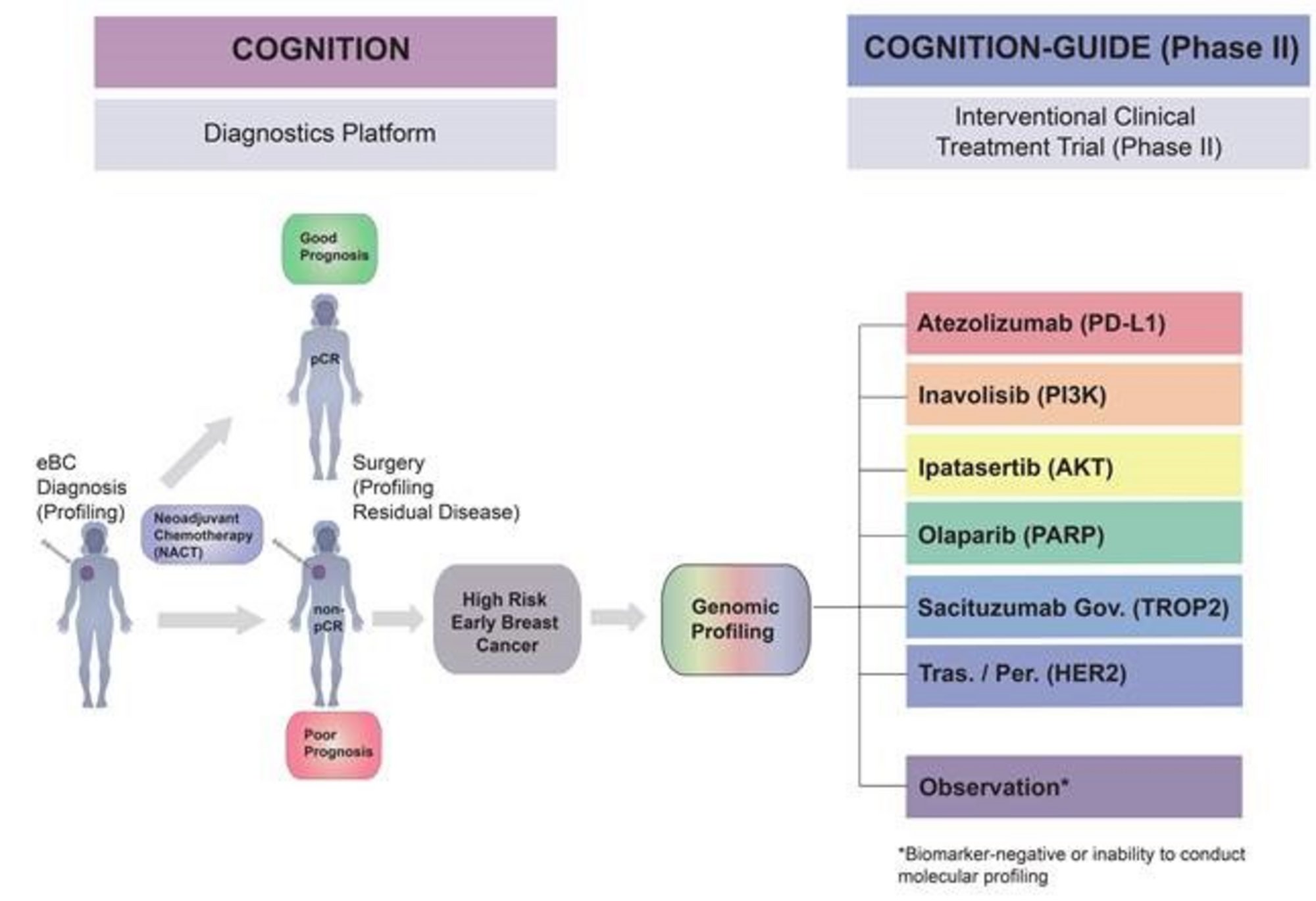
7. Biomarker-based, personalised treatment with targeted substances according to the genetic tumor profile at the NCT Heidelberg (outside the Register study) in a prospective 9-armed Phase II treatment study (COGNITION-GUIDE), which is planned for the beginning of 2021.
COGNITION-GUIDE (eBC)
Genomics guided targeted post-neoadjuvant therapy in patients with early breast cancer – a multicenter, open-label, umbrella phase-II study
Patients with early breast cancer and a high-risk for relapse following neoadjuvant chemotherapy.
TNBC or HER2+ breast cancer (BC) with residual cancer burden (non-pCR), or HR+/HER2- BC with non-pCR and CPS-EG score ≥ 3, or ypN+ and a CPS-EG score ≥ 2 after neoadjuvant therapy, surgery and standard post-neoadjuvant therapy according to current German guidelines except Abemaciclib and Olaparib. Patients were identified within COGNITION (or COGNITION-MOMENT).
Main inclusion criteria:
- Female and male patients with non-metastatic early (stage I-III) breast cancer aged ≥ 18 years
- Conducted neoadjuvant chemotherapy and surgery as well as conducted standard post-neoadjuvant treatment +/- radiotherapy (standard according to German guidelines except Abemaciclib and Olaparib)
- For patients with initially triple negative (TNBC) or HER2-positive breast cancer: Non-pCR defined as other than ypT0/is ypN0
- For patients with initially hormone receptor positive and HER2-negative breast cancer: Non-pCR and CPS-EG score ≥ 3 and ypN0, or ≥ 2 and ypN+
- ECOG Performance Status ≤ 1
- Pre- or postmenopausal
Main exclusion criteria:
- Other malignancy within the last 5 years except: adequately treated non-melanoma skin cancer, curatively treated in situ cancer of the cervix, ductal carcinoma in situ (DCIS), Stage 1 grade 1 endometrial carcinoma, or other solid tumours including lymphomas (without bone marrow involvement) curatively treated with no evidence of disease for ≥ 5 year
- Persistent toxicity (≥ Grade 2 according to NCI CTCAE v5.0 caused by previous cancer therapy, excluding alopecia)
- Clinical signs of active infection (> Grade 2 according NCI CTCAE v5.0)
- History of or newly diagnosed HIV infection and immunocompromised patients; acute HAV and active HBC- and HCV-infection
Primary Endpoint
- Invasive disease-free survival (IDFS) of patients four years after surgery overall (the aim is to achieve an improvement of 10% to 80%)
Secondary Endpoints:
- overall survival
- IDFS in each study arm separately
- distant disease-free survival
- Safety including incidence of adverse events
Depending on the molecular profiling of the tumor, patients are allocated to one of six treatment arms (Atezolizumab, Inavolisib, Ipatasertib, Olaparib, Sacituzumab Govitecan, Trastuzumab/Pertuzumab) or in case of inconsive biomarker profiles or failure to conduct molecular profiling, to an observational arm. Experimental treatment duration will be 12 months and administered additionally following all standard-of-care therapies (except endocrine therapy)
Overview of Recruiting Centers
COGNITION
Heidelberg
National Center for Tumor Diseases (NCT) Heidelberg
Prof. Dr. med. Andreas Schneeweiss
Tel.: 06221 56 5959
E-Mail: Patientenzentrum2.NCT(at)med.uni-heidelberg.de
Augsburg
University Hospital Augsburg
Prof. Dr. Nina Ditsch
Tel.: 0821 400 2208
E-Mail: studien.gyn(at)uk-augsburg.de
Berlin
Charité - Universitätsmedizin Berlin, Department of Gynaecology incl. Breast Center CCM/CBF
Prof. Dr. med. Jens-Uwe Blohmer
Tel: 030 450 664485
E-Mail: brustzentrum-studienzentrum(at)charite.de
Erlangen
Friedrich-Alexander-Universität Erlangen-Nürnberg, University Hospital Erlangen, Womens Hospital
Prof. Dr. med. Peter Fasching
Tel: 09131 85 33572
E-Mail: fk-studienzentrale(at)uk-erlangen.de
Dresden
University of Technology Dresden, Medical Faculty and University Hospital Carl Gustav Carus | Gynecology and Obstetrics
Prof. Dr. med. Pauline Wimberger
Tel.: 0351 458 4223
E-Mail: studiensekretariat.gyn(at)ukdd.de
Regensburg
Clinic for Gynecology and Obstetrics of the University of Regensburg at Caritas Hospital St. Josef
PD Dr. med. Stephan Seitz
Tel: 0941 782 7512
E-Mail: FHK-Studienzentrum(at)csj.de
Stuttgart
Robert Bosch Hospital, Department of Gynecology and Obstetrics
Robert Bosch Center for Tumor Diseases
Prof. Dr. med. Matthias Schwab
Tel. 0711 8101 7761
E-Mail: NCT-Studienzentrale(at)rbk.de
Tübingen
University Hospital Tübingen
Prof. Dr. Andreas Hartkopf
E-Mail: studienzentrale.fr(at)med.uni-tuebingen.de
Ulm
University Hospital Ulm | Womens Hospital
Prof. Dr. med. Wolfgang Janni
Tel. 0731 500 58529
E-Mail: studienzentrale.ufk(at)uniklink-ulm.de
Würzburg
Womens Hospital and Polyclinic of the University Hospital
Prof. Dr. med. Achim Wöckel
E-Mail: Studien_FK(at)ukw.de
Planned Recruitment COGNITION
Essen
University Hospital Essen | Clinic for Gynecology and Obstetrics
PD Dr. med. Oliver Hoffmann, PD, Dr. med. Ann-Kathrin Bittner
Tel.: 0201 723 – 2575
E-Mail: Studien.frauenklinik(at)uk-essen.de
Köln
University Hospital Cologne | Breast Center Cologne
PD Dr. med. Wolfram Malter
Tel.: 0221 478 37567
E-Mail: frauenklinik-studienzentrale(at)uk-koeln.de
COGNITION GUIDE
Heidelberg
National Center for Tumor Diseases (NCT) Heidelberg
Prof. Dr. med. Andreas Schneeweiss
Augsburg
University Hospital Augsburg
Prof. Dr. Nina Ditsch
Berlin
Charité - Universitätsmedizin Berlin, Department of Gynaecology incl. Breast Cancer CCM/CBF
Prof. Dr. med. Jens-Uwe Blohmer
Dresden
University of Technology Dresden, Medical Faculty and University Hospital Carl Gustav Carus
Prof. Dr. med. Pauline Wimberger
Erlangen
Friedrich-Alexander-Universität Erlangen-Nürnberg, University Hospital Erlangen, Womens Hospital
Prof. Dr. med. Peter Fasching
Tübingen
University Hospital Tübingen
Prof. Dr. Andreas Hartkopf
Ulm
University Hospital Ulm, Womens Hospital
Prof. Dr. med. Wolfgang Janni
Würzburg
Womens Hospital and Polyclinic of the University Hospital
Prof. Dr. med. Achim Wöckel
Planned Recruitment COGNITION GUIDE
Essen
University Hospital Essen, Clinic for Gynecology and Obstetrics
PD Dr. med. Oliver Hoffmann, PD Dr. med. Anne-Kathrin Bitter
Köln
University Hospital Cologne | Breast Center Cologne
PD Dr. med. Wolfram Malter
Tel.: 0221 478 37567
E-Mail: frauenklinik-studienzentrale(at)uk-koeln.de
Video: Early Breast Cancer - About the COGNITION diagnostic study (in German)
Video: Christian Maurer, MD, on the COGNITION study (in German)
Video: Christian Maurer, MD, on the COGNITION GUIDE study (in German)

Information for patients (in German) *
Contact
Tel.: 06221 56-5959 or 06221 56-7985
E-Mail: Patientenzentrum2.nct(at)med.uni-heidelberg.de
Coordination

Prof. Dr. Schneeweiss
Gynecological Oncology, NCT Heidelberg/UKHD

Prof. Dr. Lichter
Molecular Genetics, DKFZ

Prof. Dr. Schlenk
Trial Center IM5 UKHD/ NCT Trial Center
Trial conducted in close collaboration between the German Cancer Research Center (DKFZ) and Heidelberg University Hospital (UKHD) as well as the National Center for Tumor Diseases (NCT) Heidelberg.
CATCH (metastatic breast cancer)
Comprehensive assessment of clinical features and biomarkers to identify patients with advanced or metastatic breast cancer for marker driven trials in humans
Precision oncology study: Molecular diagnostics for stratifying therapy of metastasised breast cancer patients
Objective
CATCH aims to create an individual genotype and biomarker profile of prognostically leading lesions for patients with advanced metastasised breast cancer by using genome-wide high-throughput tumor genome sequencing.
By employing this strategy, prognostic and predictive markers, therapy-relevant driver mutations and biomarkers are identified to optimise the treatment decision for targeted substances.
Treatment outside of the register study occurs in (1) independent phase I-III clinical studies, (2) in the licenced indication or as (3) off-label use.
Therapeutic resistance towards conventional therapy is especially attributed to high intra- and inter-tumoral heterogeneity. Furthermore, nowadays only a limited amount of targeted substances for advanced breast cancer is available and diagnostic procedures routinely do not contain comprehensive biomarker evaluation.
Strategy:
Within the CATCH study predictive biomarkers and drug targets are evaluated using integrative comprehensive tumor genome analysis (whole-genome and transcriptome sequencing). Within the register study all procedures, from inclusion to biomaterial extraction to prioritising targeted therapies, are implemented in clinical practice. With the gained biological information, the practising oncologist at NCT is able to identify patients who profit from innovative targeted treatment modalities.
Treatment occurs at the discretion of the practising oncologist 1) within independent Phase I/-IV AMG studies, 2) in the authorised indication or (3) as off-label use.
Endpoints:
PFS2 / PFS2 Ratio: Progression-free survival (PFS) during molecular stratified therapy in comparison to PFS during immediately preceding standard therapy of the individual patient.
Inclusion criteria:
- mBC patients (all subtypes) after initial diagnosis or at the time of progression
- life expectancy > 6 months
- ECOG ≤ 2
- Possibility to extract fresh tissue of the leading prognostic lesion
Exclusion criteria:
- Impossibility to extract fresh tissue
The diagnostic platforms follow a standardised workflow:
1. Information / enrolment at the NCT Heidelberg or any other participating study center
2. Acquisition of biomaterial (tissue + blood) for molecular analyses in the course of standard routine procedures or as a study specific measure
- Tumor tissue: Fresh tissue samples (no FFPE if possible) as pre-requesite for downstream molecular-genetic analyses
- Blood withdrawal (comparative sample of normal body cells to define tumor-specific genetic alterations)
3. Evaluation tumour cell content fresh tissue sample (minimum content 20 % tumor cells) and tumor infiltrating lymphocytes
4. Molecular Analyses: Tumor-Genome-Sequencing (DKFZ Heidelberg) / Next Generation Sequencing
- Analyte-Isolation (DNA, RNA, Protein, Sample Processing Lab, DKFZ)
- Sample preparation for high-throughput sequencing (Genomics Core Facility, DKFZ)
- Method: whole-genome/ whole-exome and transcriptome sequencing
5. Discussion of molecular-genetic findings within the interdisciplinary molecular tumor board
- Participants: Oncologists, bioinformaticians, molecular biologists, pathologists, human geneticists
- Prioritisation of therapy-relevant gene alterations and molecular targets
- Generating a therapy plan combined in a molecular report
6. Discussion of molecular-genetic findings and consideration of possible treatment options with the patient
Overview of Recruiting Centers
CATCH
Heidelberg
National Center for Tumor Diseases (NCT) Heidelberg
Prof. Dr. med. Andreas Schneeweiss
Tel.: 06221 56 5959
E-Mail: Patientenzentrum2.NCT(at)med.uni-heidelberg.de
Berlin
Charité - Universitätsmedizin Berlin, Department of Gynaecology incl. Breast Center CCM/CBF
Prof. Dr. med. Jens-Uwe Blohmer
Tel: 030 450 664485
E-Mail: brustzentrum-studienzentrum(at)charite.de
Dresden
University of Technology Dresden, Medical Faculty and University Hospital Carl Gustav Carus | Gynecology and Obstetrics
Prof. Dr. med. Pauline Wimberger
Tel.: 0351 458 4223
E-Mail: studiensekretariat.gyn(at)ukdd.de
Augsburg
University Hospital Augsburg
Prof. Dr. Nina Ditsch
Tel.: 0821 400 2208
E-Mail: studien.gyn(at)uk-augsburg.de
Ulm
University Hospital Ulm | Womens Hospital
Prof. Dr. med. Wolfgang Janni
Tel. 0731 500 58529
E-Mail: studienzentrale.ufk(at)uniklink-ulm.de
Tübingen
University Hospital Tübingen
Prof. Dr. Andreas Hartkopf
E-Mail: studienzentrale.fr(at)med.uni-tuebingen.de
Erlangen
Friedrich-Alexander-Universität Erlangen-Nürnberg, University Hospital Erlangen, Womens Hospital
Prof. Dr. med. Peter Fasching
Tel: 09131 85 33572
E-Mail: fk-studienzentrale(at)uk-erlangen.de
Würzburg
Womens Hospital and Polyclinic of the University Hospital
Prof. Dr. med. Achim Wöckel
E-Mail: Studien_FK(at)ukw.de
Stuttgart
Robert Bosch Hospital, Department of Gynecology and Obstetrics
Robert Bosch Center for Tumor Diseases
Prof. Dr. med. Matthias Schwab
Tel. 0711 8101 7761
E-Mail: NCT-Studienzentrale(at)rbk.de
Regensburg
Clinic for Gynecology and Obstetrics of the University of Regensburg at Caritas Hospital St. Josef
PD Dr. med. Stephan Seitz
Tel: 0941 782 7512
E-Mail: FHK-Studienzentrum(at)csj.de
Essen
University Hospital Essen | Department of Internal Medicine (Tumor Research), Hematology and Oncology
PD Dr. med. Anja Welt
Tel. 0201 723 85140
E-Mail: studienkoordination.NCT(at)uk-essen.de
Köln
University Hospital Cologne | Breast Center Cologne
PD Dr. med. Wolfram Malter
Tel.: 0221 478 37567
E-Mail: frauenklinik-studienzentrale(at)uk-koeln.de
Video: Advanced breast cancer - About the CATCH diagnostic study (in German)
Video: Prof. Andreas Schneeweiss, MD, on the CATCH study (in German)
Contact
Tel.: 06221 56-5959 or 06221 56-7985
E-Mail: Patientenzentrum2.nct@med.uni-heidelberg.de

Prof. Dr. Schneeweiss
Gynecological Oncology, NCT Heidelberg/UKHD

Prof. Dr. Lichter
Molecular Genetics, DKFZ
The diagnostic trials are carried out in close cooperation between the University Hospital, the German Cancer Research Center (DKFZ) and the Molecular Diagnostics Program at NCT Heidelberg.
Further Clinical Trials
The Division of Gynecological Oncology conducts numerous further diagnostic, treatment, observational and clinical trials.