Tumorimmunologie
Leitung: Prof. Dr. Dirk Jäger
Im Kampf gegen Krebs stellt das Immunsystems ein zweischneidiges Schwert dar: Es schützt vor Tumorentstehung und Metastasierung (Immunüberwachung), fördert aber auch die Tumorbildung, indem es anti-tumorale Immunantworten hemmt, z.B. über Einflussnahme auf die Tumorumgebung. Unser Fokus liegt auf der Erforschung systemischer und lokaler immunologischer Effekte in Krebspatient:innen. Das Immunsystem beeinflusst die Wirksamkeit von Therapien und Interventionen. Eine detailliertere Betrachtung der beteiligten immunologischen Prozesse eröffnet daher die Tür für neue Interventionen.
Ein besonderer Schwerpunkt unserer Forschung liegt auf der Analyse immunologischer Interaktionen zwischen Tumorzellen und Wirts- oder Immunzellen in der Mikroumgebung und Peripherie des Tumors, um prädiktive Biomarker zu identifizieren und neuartige immuntherapeutische Ansätze für solide Tumoren zu entwickeln (z. B. TCR-T-Zellen, CAR-T-Zellen, bispezifische Antikörper und Peptidimpfstoffe). Organoid- und Gewebekulturen werden als (autologe) Tumormodellsysteme für die in vitro Testung von Zellprodukten und antikörperbasierten Verbindungen eingesetzt und ebnen den Weg für frühe klinische Studien (Phase I/II) zur Erforschung neuartiger (Kombinations-)Therapien.
Die Tumorimmunologie Gruppe arbeitet eng mit Prof. Jägers Klinischer Kooperationseinheit „Angewandte Tumorimmunität" am DKFZ sowie mit dem NCT-geförderten Immuntherapie Programm (geleitet von Prof. Dr. Angelika Riemer (DKFZ) und Prof. Dr. Dirk Jäger) zusammen.
Mit unserem starken Fokus auf Immuntherapie sind wir Teil des "Cancer Vaccine Collaborative” des Cancer Research Institute (New York) und ein Partner des Ludwig Institute for Cancer Research (New York).

© Marten Meyer, Erstellt mit BioRender.com
Research topics
Cellular Therapies
Adoptive T cell therapy using genetically engineered T cells expressing a chimeric antigen receptor (CAR) has revolutionized the treatment modalities in leukemia and lymphoma for more than a decade now. However, the translation of the successes to solid tumor diseases is rather slow. Within our team, we are dedicated to the development and manufacturing of novel and safe CARs that can be applied in the clinics. For instance, we have developed two novel CAR-T cell products for the use in breast cancer patients that target the cancer testis antigen NY-BR-1 and prevent cross-reactivity against healthy brain tissue. Our team analyzed both for efficiency and safety in vitro and in vivo in a xenotransplant and target transgenic mouse model.
In close collaboration with Dr. Richard Harbottle (DKFZ, DNA Vector Lab), we have developed a novel DNA-plasmid-based vector system for the GMP-grade manufacturing of CAR‑T cells that makes the use of (lenti)viral vectors dispensable. These vectors, which we named nS/MARt, do not possess the risk of genotoxicity but confer the same characteristics as viral vectors, e.g. stable expression (Bozza M et al, 2021, SciAdv, 7(16):eabf1333). As desired side effects, this novel manufacturing method will reduce overall costs for CAR-T cell production and allows the establishment of a de-centralized manufacturing process.
As an accompanying project, we are developing novel, specific CAR inhibitors that can temporarily impair their function and therefore maybe useful to lower clinical side effects. These inhibitors are based on non-pathological AAV-particles that present specific CAR-epitopes on their surface. Currently, we are validating our patented technology (WO/2019/043081) by identification of matching pairs of CARs and CARAAVs.
In a joint effort together with the CCU “Applied Tumor Immunity” (DKFZ) we try to accelerate the identification of de novo, tumor antigen-specific CAR constructs to be used in personalized cancer immunotherapy. To achieve this goal, a unique cellular CAR library screening platform is generated by taking advantage of novel nanoSMAR vectors and the Lightning System, a single-cell microfluidic system from Berkeley Lights.
Contact: Patrick Schmidt, PhD - patrick.schmidt(at)nct-heidelberg.de
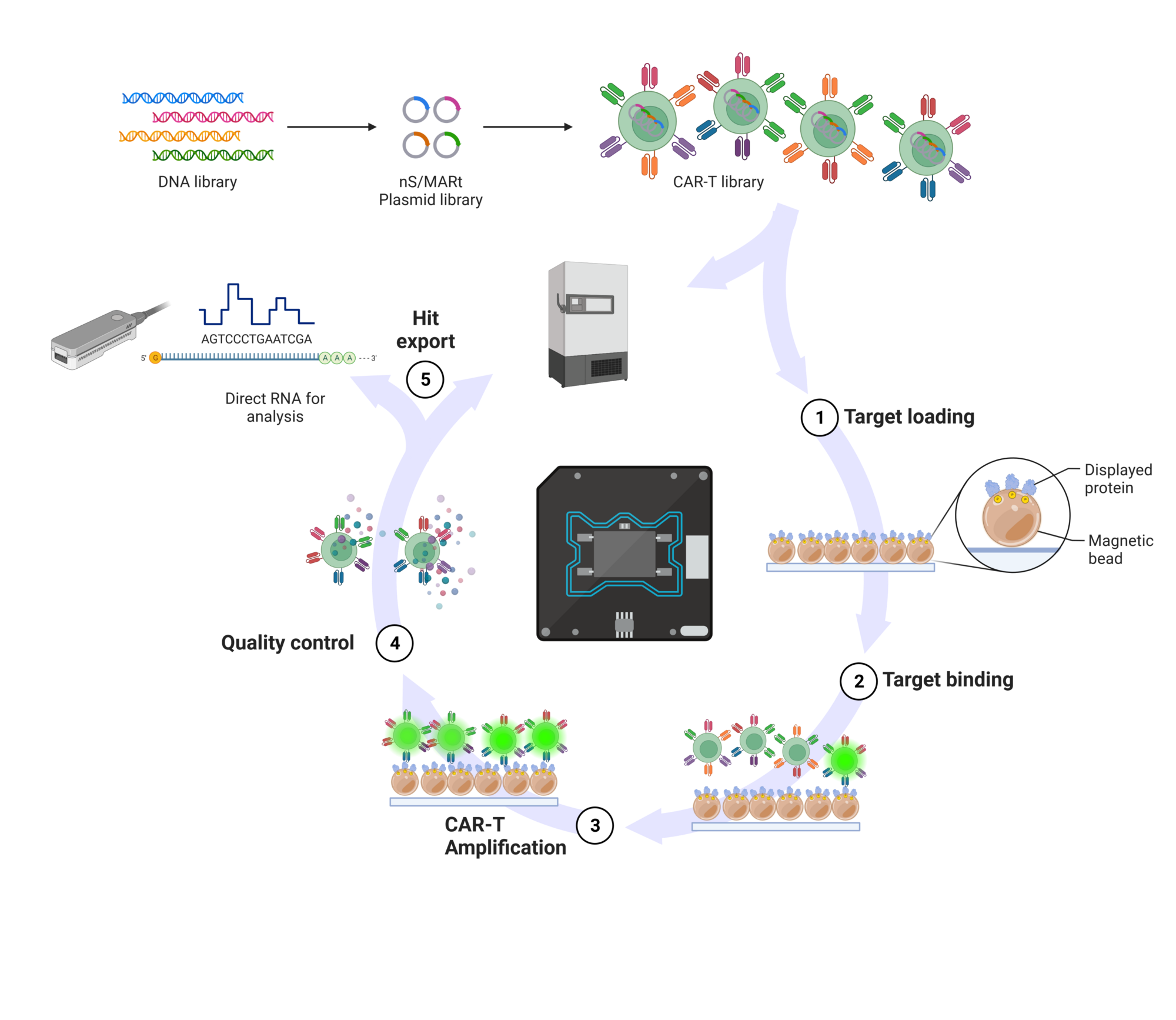
© Patrick Schmidt, Created with BioRender.com.
We aim for the development and routine application of personalized TCR gene-engineered adoptive T cell therapies (ACT) targeting individual- as well as shared patient-derived neoepitopes. Based on our implemented state-of-the-art tumor mutagenome analysis as well as in silico T cell epitope prediction pipeline that is combined with our highly sensitive peptide-loaded MHC-multimer-guided antigen-specific T cell discovery workflow (CCU “Applied Tumor Immunity” (DKFZ), Antigen Presentation and T/NK Cell Activation, PD Dr. Momburg), we have already identified and validated several neoepitope-specific TCRs across various solid tumor identities. This TCR discovery workflow is further augmented by our own robust pMHC in-house production platform that outperforms commercially available reagents and allows us to provide complete coverage of the individual patients’ HLA allotypes. By incorporating DNA-barcoded pMHC-I multimer reagents into the workflow, we link multiplexed tumor antigen recognition with their associated TCR repertoire and T cell differentiation phenotype at single cell resolution, which further improves the selection of TCR / antigen pairs for validation prior to their usage in an ACT.
The PIKT project is a pilot project funded by the Dietmar Hopp foundation for the development of personalized cellular immunotherapies for solid tumor diseases. The scope of the project is the identification and cloning of individual neoepitope specific TCRs and the manufacturing of clinical-grade transgenic TCR-T cell products as a proof of concept. For TCR discovery, the above pipeline is applied. We have screened more than 35 cases and so far and T cell receptors for nine cases were successfully identified, cloned and validated.
To deepen our knowledge about T cell (dys)function, a panel of analytical methods to determine T cell fitness of patients before and after T cell therapy by multiparametric flow cytometry has been established in close collaboration with Dr. Paula Roberti, CCU “Applied Tumor Immunity” (DKFZ). In an individual case, the association between a high T cell fitness signature in the infusion product and long-term persistence of TCR-T cells in the patient with sustained reactivity to the tumor target ex vivo could be shown. In addition to T cell fitness, other tumor-intrinsic resistance mechanisms are explored with the goal to not only find resistance mechanisms to cellular therapies, but also to develop new tools to overcome them.
Contact:
- Marten Meyer, PhD - marten.meyer(at)dkfz-heidelberg.de
- Maria Paula Roberti, PhD - mariapaula.roberti(at)nct-heidelberg.de
- Inka Zörnig, PhD - inka.zoernig(at)nct-heidelberg.de
We have established manufacturing protocols for the GMP production of CAR-T cells and transgenic TCR-T cells using the semi-automated manufacturing platform CliniMACS Prodigy from Miltenyi Biotec. Protocols are available for the lentiviral vector based production as well as for production using DNA-plasmid vectors. With strong support by the Dietmar Hopp Foundation, we envision to establish a TCR discovery lab and GMP manufacturing facility for cellular medical products in 2025. The so called “Center for Innovative Therapies” will harbor 320 sqm of clean room space classified in levels B, C and D in which production of individualized tumor vaccines and patient specific cell infusions will be possible. All rooms will be equipped with state-of-the-art devices in order to be able to perform any current manufacturing process possible. A dedicated and trained team will constantly establish SOPs and realize the regulatory prerequisites. This will enable us to develop innovative therapy approaches and produce study drugs for Phase I trials at the NCT, the UKHD, for partner sites within the NCT network as well as to serve as manufacturer for approved cellular drugs.
Contact: Patrick Schmidt, PhD - patrick.schmidt(at)nct-heidelberg.de
For the testing and individualized validation of CAR/TCR-T cell approaches, tissue co-cultures/organoids are applied with subsequent in depth analyses of the cell products, co-culture supernatants and tissue/organoids by multimarker FACS, IHC, virtual microscopy, and semi-automated image analyses.
Contact:
- Patrick Schmidt, PhD - patrick.schmidt(at)nct-heidelberg.de
- Inka Zörnig, PhD - inka.zoernig(at)nct-heidelberg.de
Biomarker trials and clinical studies
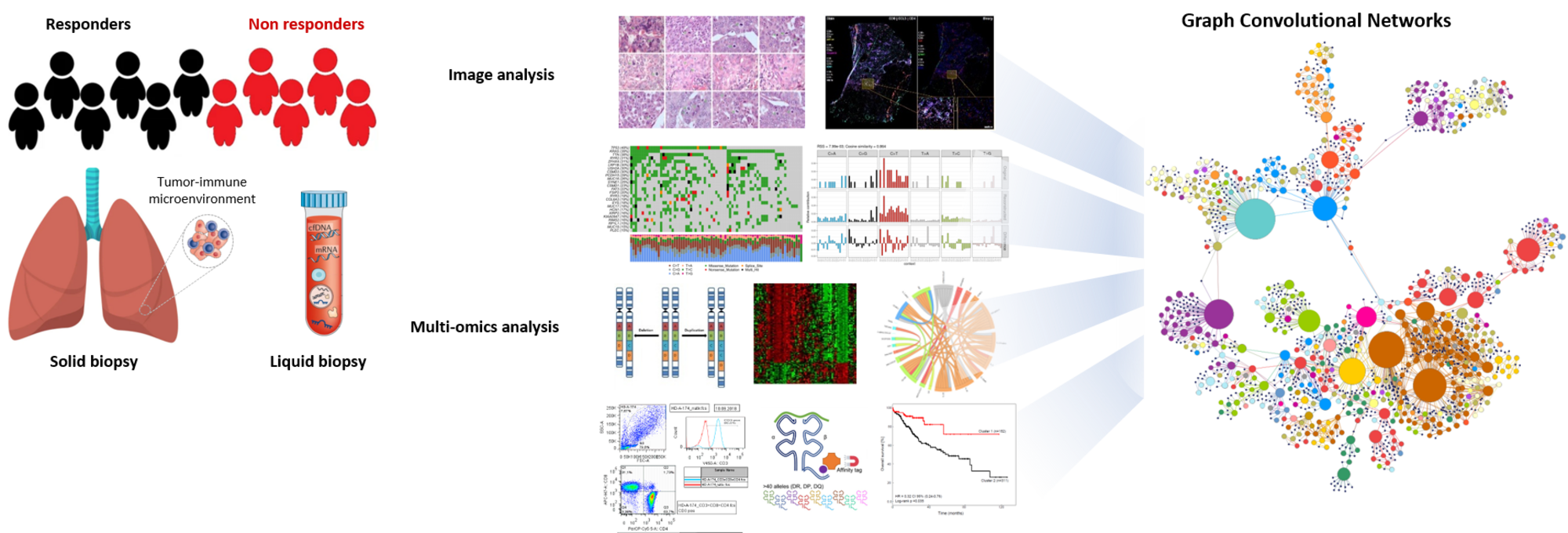
© Pornpimol Charoentong
The PROMISE trial (PRedictability of Outcome based on iMmunological sIgnatureS in lung cancEr – an explorative investigation) is a collaborative exploratory biomarker study with the Thoraxklinik, the UKHD and the DKFZ, funded by the Dietmar Hopp foundation. The trial was initiated in 2018 and was open for recruitment until summer 2022. Overall, 136 patients have been enrolled in the study.The trial focusses on the identification of predictive markers or signatures for response and resistance to current standard of care treatment in stage IV non-small cell lung cancer (NSCLC) patients. Biomaterials, such as tissue, serum, plasma, PBMCs, breath condensates and stool samples, are systematically collected longitudinally and will be analyzed by e.g. WGS/WES, epigenomics, PanelSeq, RNASeq, Nanostring, metagenomics, metabolomics, immunohistochemistry and virtual microscopy with semi-automated image analyses, multiplex Cytokine/Chemokine assays, mass spectrometry, RAMAN spectroscopy and flow cytometry for naïve/memory/effector immune cell phenotyping. Furthermore, we are analyzing the TCR repertoire in responders/non-responders in our clinical cohorts by deep sequencing of TCR variable beta (Vβ) (TCRB) chain. This will allow us to evaluate and measure the clonality and diversity of T cells that can arise in response to tumor-associated antigens and to investigate whether these changes could be predictive markers of response.
Due to the advancement in high-throughput technologies, we incorporate heterogeneous data from multiple technologies to improve our understanding of the molecular dynamics associated with biological processes. Pipelines for multi-omics data analysis comprising MHC I/II epitope prediction and V(D)J enrichment immune profiling analysis have been set up. Further, we are developing machine- and deep learning approaches on an in-house generated graph database enabling us to extract relevant biological interpretations and to identify novel relationships by integrating high-dimensional data. For an in-depth understanding of the “individual disease” and for the identification of predictive biomarkers, an integrated analysis of the multiomics (Charoentong P et al, 2017, Cell Rep, 18(1):248-62) and wet lab data is performed.
Contact:
- Pornpimol Charoentong, PhD - pornpimol.charoentong(at)nct-heidelberg.de
- Inka Zörnig, PhD - inka.zoernig(at)nct-heidelberg.de
The translational research program of the NEOMUN trial (“NEOadjuvant anti-PD-1 imMUNotherapy in resectable NSCLC: The NEOMUN Trial”) is supported by NCT POC trial funding and is carried out in the CCU Applied Tumor Immunity (DKFZ) /NCT TME Unit in collaboration with PD Dr. M. Eichhorn and colleagues (Surgery Department, Thoraxklinik Heidelberg). A major aim is the analysis of possible therapy-associated changes in different tumor regions (e.g. core vs. border), lymph nodes or healthy lung tissue and the potential identification of predictive markers.
Contact:
- Pornpimol Charoentong, PhD - pornpimol.charoentong(at)nct-heidelberg.de
- Inka Zörnig, PhD - inka.zoernig(at)nct-heidelberg.de
The IReP trial (“Immune Response Prediction” trial; funded by the Dietmar Hopp Foundation) is an exploratory study evaluating the potential of immune signature profiling for predicting response to neoadjuvant chemoimmunotherapy in patients with resectable stage II/III non-squamous NSCLC. An in depth analysis of the tumor microenvironment and genomic alterations is performed in pre- and post-treatment tissue and data is correlated with response as defined by Major pathologic response. The trial is performed in collaboration with PD Dr. M. Eichhorn (Department of Surgery, Thoraxklinik).
Contact:
- Pornpimol Charoentong, PhD - pornpimol.charoentong(at)nct-heidelberg.de
- Inka Zörnig, PhD - inka.zoernig(at)nct-heidelberg.de
To bring Fecal Microbiota Transfer (FMT) therapy to the standard of care, the open challenge is to identify cancer patients at risk of gut-related therapy failure who are amenable to FMT. We are currently collaborating with clinicians and scientists at UKHD and NCT (Dr. C. Rauber) to conduct the "Mucosome" study (S-633/2021) with the aim of identifying gut biomarkers in patients with extraintestinal cancers that can be used for the selection of patients who will potentially benefit from FMT and to identify stool donors that can be used pharmacologically in a tailored donor-recipient approach in cancer therapy.
Contact: Maria Paula Roberti, PhD - mariapaula.roberti(at)nct-heidelberg.de
In multifocal hepatocellular carcinoma, the recently introduced first-line therapy Atezolizumab/Bevacizumab leads to an objective response rate of only 27%, which is superior to previous first-line therapies but highlights the urgent need for further therapeutic improvement. Together with Dr. C. Rauber (UKHD) and Dr. M. Dill (UKHD/DKFZ), who conduct "The FLORA trial: Fecal Microbiota Transfer in Liver Cancer to Overcome Resistance to Atezolizumab/Bevacizumab” Dr. M. Paula Roberti and the CCU team will perform the accompanying translational research program. Part of the “NCT Proof-of-Concept Trial Program” within the framework of Liver Cancer Center Heidelberg (LCCH), FLORA is a randomized, single-blind, placebo-controlled phase II clinical trial, which aims to assess immunogenicity and safety of FMT in combination with standard of care immunotherapy in advanced hepatocellular carcinoma.
Contact: Maria Paula Roberti, PhD - mariapaula.roberti(at)nct-heidelberg.de
The comprehensive analysis of tumor-host cell interactions, especially focusing on immune cells and the spatial analysis of the tumor microenvironment by immunohistochemistry (IHC), multiplex analyses, virtual microscopy and semi-automated image analysis is another main objective of the division. The molecular mechanisms underlying the clinical impact and the causes of the observed differences in the immunological tumor micro-milieu are not yet fully understood. Therefore, the dedicated analysis of the tumor compartments (stroma, epithelium, invasion front, and surrounding tissue) with respect to localization of immune cells and cytokine profiling in the light of the clinical course will yield a better image of the complex relations and eventually support the development of new drugs and targeted treatment approaches for patients. Together with the CCU Applied Tumor Immunity (DKFZ), the NCT TME Unit and the NCT Unit for „Applied Tumor Therapy and Combinational Strategies“, collaboration projects and clinical studies, especially biomarker trials such as PROMISE, NEOMUN and also programs such as CATCH, COGNITION and MASTER are supported. Further, the group is closely interacting with other units of the NCT Immunotherapy Program, which has been established to support drug- and target-development for novel immunotherapies (e.g. antibody-based strategies, cellular approaches and vaccines). This includes pre-clinical testing in cell culture, in human tissue model systems, organoids as well as small animal models and subsequent in-depth analysis of the tumor microenvironment.
Contact:
- Inka Zörnig, PhD - inka.zoernig(at)nct-heidelberg.de
Bispecific antibodies
Although T cell-recruiting CD3-binding bispecific monoclonal antibodies (BiMAb) are clinically effective for hematologic malignancies, the success of BiMAb targeting solid tumor-associated antigens (TAA) in carcinomas remains poor. We reason that co-stimulatory BiMAb in combination with anti-CD3 BiMAb would boost T cell responses, facilitating the targeting of weakly or heterogeneously expressed tumor antigens. We have analyzed anti-TAA–anti-CD3/anti-CD28 BiMAb, recognizing breast cancer antigens including HER2, EGFR, CEA, and EpCAM. Moreover, bifunctional fusion proteins with tumor necrosis factor ligand (TNFL) superfamily members (4-1BBL, OX40L, CD70, TL1A) were tested. The functional activity of our bispecific antibodies was evaluated in cell-based assays. The anti-tumor activity was confirmed in a patient-derived 3D spheroid model using pleural effusions of breast cancer patients. Only in the presence of tumor cells, anti-CD3 BiMAb activated T cells and induced cytotoxicity in vitro, indicating strict dependence on cross-linking. Combination with anti-CD28 or TNFL bispecifics enhanced T cell proliferation, activation marker expression, cytokine secretion and tumor cytotoxicity, thereby reducing the minimally required dose to achieve T cell activation. Effective co-stimulation could be achieved by targeting a second breast cancer antigen, or by targeting fibroblast activation protein (FAP) expressed on another target cell. Immune checkpoint inhibitors further augmented BiMAb mediated co-stimulation. In patient-derived spheroids, co-stimulatory BiMAb were essential for activation of tumor-infiltrating lymphocytes and cytotoxic anti-tumor responses against breast cancer cells. Combining stimulatory and co-stimulatory BiMAb may have promising clinical application in the treatment of solid tumors.
We investigate bispecific antibodies that redirect T cells to the tumor neo-vasculature. Using VEGFR2, TIE2 and PD-L1 as target antigens expressed by proliferating endothelial cells (EC), we found that bispecific antibodies addressing either CD3 or CD28 on T cells are effective in recruiting T cells towards EC leading to a profound on-site T cell activation as well as upregulation of adhesion molecules of resting EC. This results in an augmented transendothelial migration in vitro and subsequent tumor cell killing.
Together with partners in Israel, we engineer and test novel bispecific antibodies that target NK cells towards cancer cells. Such antibodies trigger NK cells through the activating NKp46 receptor after cross-linking with, e.g., HER-2+ tumor cells and elicit tumor antigen-specific NK cytotoxicity against MHC-I-positive tumor targets. Bispecific antibodies that mediate NK cell immune checkpoint inhibition at the tumor site are in development.
We have engineered tetravalent IgG-like TCR-antibody fusion proteins that are able to redirect NK cells towards tumor cells expressing particular peptide-MHC-I complexes recognized by respective TCRs. NK cells are recruited and activated through single-chain antibodies (scFv) binding NKp46 or CD16. Furthermore, TCR-anti-CD3 scFv fusion proteins are successfully employed to elicit T cell-mediated cytotoxicity against pMHC-I-presenting tumor cells. Multimeric formats are under development in order to increase the avidity of TCR–HLA-I interactions.
Recombinant fusion proteins of MHC class I molecules loaded with viral peptides and single-chain antibodies recognizing tumor-associated antigens such as EGFR or HER-2 ("HistoMAb") enable the recruitment to solid tumors of virus-peptide specific T cells with specificity for the particular peptide MHC-I complex. After showing in vitro proof-of-principle for HistoMAb with human human tumor antigen reactivity, we are in the process of establishing mouse tumor models to examine the in vivo efficacy of murine surrogate HistoMAb and xenograft models together with adoptive human T cell transfer. Furthermore, HistoMAb molecules with specificity for B-cell, T-cell and myeloid leukemia antigens will be systematically investigated.
Contact:
- Frank Momburg, PD MD - f.momburg(at)dkfz-heidelberg.de
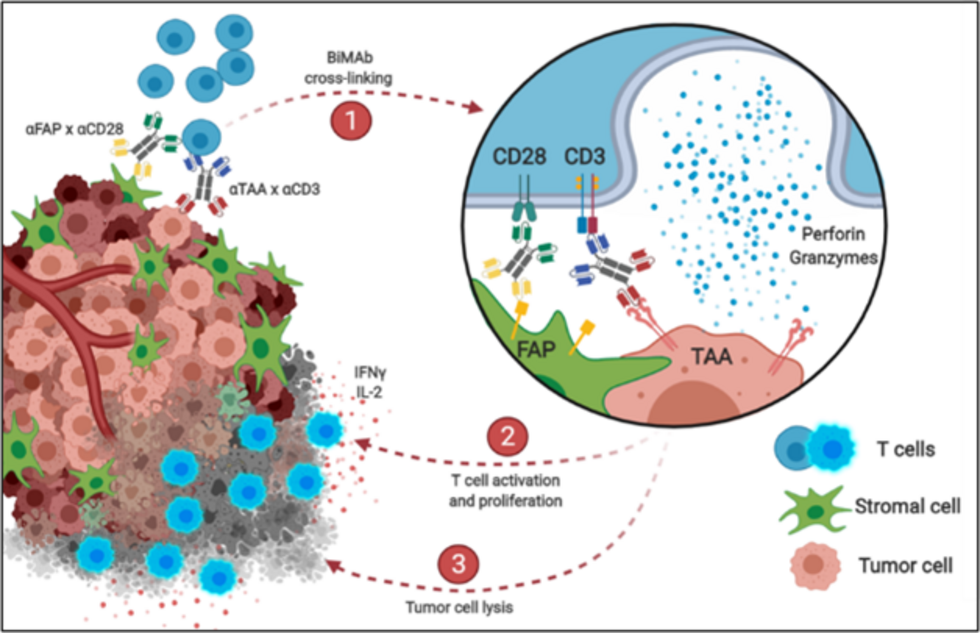
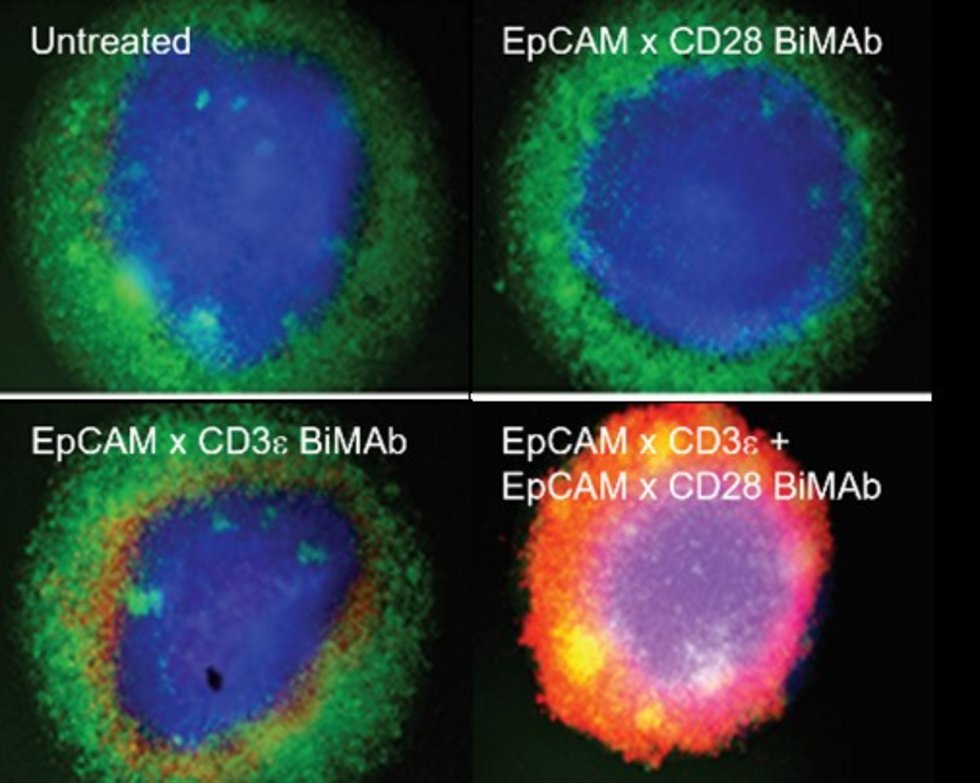
Bispecific antibodies mediate killing of breast cancer cells by T cells. Left: Graphical presentation of stimulation and co-stimulation of T cells by bispecific antibodies (BiMAb). In the “split” co-stimulation approach the co-stimulatory anti-CD28 bispecific antibody recognizes a tumor cell surface antigen that is different from the antigen targeted by the stimulatory anti-CD3 bispecific antibody. The former antigen can also be expressed by an adjacent stromal cell. Right: Strong tumor cell cytotoxicity is elicited by co-stimulation. Breast cancer cell organoids (blue) are cocultured with T cells (green) in the presence of BiMAb. If stimulatory and co-stimulatory BiMAb are simultaneously added, T cells efficiently invade the organoid and kill tumor cells (lower right, cell death indicated by red fluorescent dye). From: Warwas et al., Front. Immunol. 12:719116 (2021).
© Frank Momburg
- Jäger D, Berger A, Tuch A, Luckner-Minden C, Eurich R, Hlevnjak M, Schneeweiss A, Lichter P, Aulmann S, Heussel CP, Fremd C, Harbottle R, Zörnig I, Schmidt P. Novel chimeric antigen receptors for the effective and safe treatment of NY-BR-1 positive breast cancer. Clin Transl Med 14(7):e1776 (2024). DOI: 10.1002/ctm2.1776.
- Meyer M, Parpoulas C, Barthélémy T, Becker JP, Charoentong P, Lyu Y, Börsig S, Bulbuc N, Tessmer C, Weinacht L, Ibberson D, Schmidt P, Pipkorn R, Eichmüller SB, Steinberger P, Lindner K, Poschke I, Platten M, Fröhling S, Riemer AB, Hassel JC, Roberti MP, Jäger D, Zörnig I, Momburg F. MediMer: a versatile do-it-yourself peptide-receptive MHC class I multimer platform for tumor neoantigen-specific T cell detection. Front Immunol 14:1294565 (2024). DOI: 10.3389/fimmu.2023.1294565.
- Warwas KM, Meyer M, Gonçalves M, Moldenhauer G, Bulbuc N, Knabe S, Luckner-Minden C, Ziegelmeier C, Heussel CP, Zörnig I, Jäger D, Momburg F. Co-Stimulatory Bispecific Antibodies Induce Enhanced T Cell Activation and Tumor Cell Killing in Breast Cancer Models. Front Immunol 12:719116 (2021). DOI: 10.3389/fimmu.2021.719116.
- Eichhorn F, Klotz LV, Kriegsmann M, Bischoff H, Schneider MA, Muley T, Kriegsmann K, Haberkorn U, Heussel CP, Savai R, Zoernig I, Jaeger D, Thomas M, Hoffmann H, Winter H, Eichhorn ME. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: First clinical experience. Lung Cancer. 153:150-157 (2021). doi: 10.1016/j.lungcan.2021.01.018.
- Bozza M, De Roia A, Correia MP, Berger A, Tuch A, Schmidt A, Zörnig I, Jäger D, Schmidt P, Harbottle RP. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Sci Adv 7(16):eabf1333 (2021). DOI: 10.1126/sciadv.abf1333.
- Ran T, Eichmüller SB, Schmidt P, Schlander M. Cost of decentralized CAR T-cell production in an academic nonprofit setting. Int J Cancer. 147(12):3438-3445 (2020). DOI: 10.1002/ijc.33156.
- Rölle A, Meyer M, Calderazzo S, Jäger D, Momburg F. Distinct HLA-E Peptide Complexes Modify Antibody-Driven Effector Functions of Adaptive NK Cells. Cell Rep 24:1967-76.e4 (2018). DOI: 10.1016/j.celrep.2018.07.069.
- Michels J, Becker N, Suciu S, Kaiser I, Benner A, Kosaloglu-Yalcin Z, Agoussi S, Halama N, Pawlita M, Waterboer T, Eichmüller SB, Jäger D, Eggermont AMM, Zörnig I. Multiplex bead-based measurement of humoral immune responses against tumor-associated antigens in stage II melanoma patients of the EORTC18961 trial. Oncoimmunology. 12;7(6):e1428157 (2018). DOI: 10.1080/2162402X.2018.1428157.
- Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, Suetterlin T, Brand K, Krauss J, Lasitschka F, Lerchl T, Luckner-Minden C, Ulrich A, Koch M, Weitz J, Schneider M, Buechler MW, Zitvogel L, Herrmann T, Benner A, Kunz C, Luecke S, Springfeld C, Grabe N, Falk CS, Jäger D. Tumoral immune cell exploitation in colorectal cancer liver metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 29(4):587-601 (2016). DOI: 10.1016/j.ccell.2016.03.005.
- Gnjatic S, Ritter E, Büchler MW, Giese NA, Brors B, Frei C, Murray A, Halama N, Zörnig I, Chen YT, Andrews C, Ritter G, Old LJ, Odunsi K, Jäger D. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A 107(11):5088-93 (2010). DOI: 10.1073/pnas.0914213107.
- Seil I, Frei C, Sültmann H, Knauer SK, Engels K, Jäger E, Zatloukal K, Pfreundschuh M, Knuth A, Tseng-Chen Y, Jungbluth AA, Stauber RH, Jäger D. The differentiation antigen NY-BR-1 is a potential target for antibody-based therapies in breast cancer. Int J Cancer. 120(12):2635-42 (2007). DOI: 10.1002/ijc.22620.
- Jäger D, Stockert E, Güre AO, Scanlan MJ, Karbach J, Jäger E, Knuth A, Old LJ, Chen YT. Identification of a tissue-specific putative transcription factor in breast tissue by serological screening of a breast cancer library. Cancer Res 61(5):2055-2061 (2001).

Prof. Dr. Dirk Jäger
Gruppenleitung 'Tumorimmunologie' und Leitung des NCT Programms ‚Immuntherapie‘, NCT Heidelberg
Direktor der Medizinischen Onkologie, Universitätsklinikum Heidelberg (UKHD)
Direktor des Nationalen Centrums für Tumorerkrankungen (NCT) Heidelberg
Leitung der KKE Angewandte Tumorimmunität und der AG Angewandte Tumorimmunität, DKFZ Heidelberg
Sekretariat Medizinische Onkologie
Birgit Eberle
T: 06221 56-7229
E: birgit.eberle(at)nct-heidelberg.de
Teamassistenz Klinische Kooperationseinheit
Yuk Ching Chan
E: yukching.chan(at)nct-heidelberg.de
Wissenschaftliche Koordination Medizinische Onkologie
Dr. Lea Schweßinger
E: lea.schwessinger(at)nct-heidelberg.de
Labor- und Forschungskoordination Tumorimmunologie
Dr. Inka Zörnig
E: inka.zoernig(at)nct-heidelberg.de
Group Head
Prof. Dr. Dirk Jäger
Head of 'Tumor Immunology' and Head of NCT Program 'Immunotherapy', NCT Heidelberg
Director of the Medical Oncology Department, Heidelberg University Medical Center (UKHD)
Director of the National Center for Tumor Diseases (NCT) Heidelberg
Head of the CCU Applied Tumor Immunity Unit and the Applied Tumor Immunity Group (DKFZ)
Secretariat Medical Oncology
Birgit Eberle
Tel: +49 6221 56-7229
Email: birgit.eberle(at)nct-heidelberg.de
Research Associates
Liam Bartels (DKFZ)
Position: Researcher
Area of expertise: Computational pathology, Machine learning, Medical sample imaging
Email: liam.bartels(at)dkfz-heidelberg.de
Daniel Browne
Position: IT System Administrator
Email: daniel.browne(at)bioquant.uni-heidelberg.de
Marion Camus (DKFZ)
Position: Medical PhD Student
Title: „Development of bispecific antibodies and soluble T-cell receptors for the treatment of solid tumors in organoid models for the precision-based immuno-oncology.“
Email: marion.camus(at)dkfz-heidelberg.de
Pornpimol Charoentong, PhD
Position: Senior Research Associate, Bioinformatician
Area of expertise: Genomics and Bioinformatics, Computational System Biology, Biostatistics, Management of biological databases
Email: Pornpimol.Charoentong(at)nct-heidelberg.de
Marcia Figueiredo Goncalves, Dr. sc. Hum. (DKFZ)
Position: Senior Research Associate
Area of expertise: Tumor Immunology, Antibodies
Email: m.goncalves(at)dkfz-heidelberg.de
Kyra Fuchs
Position: Research Associate
Email: kyra.fuchs(at)dkfz-heidelberg.de
Niels Grabe, PhD (Prof. Dr.)
Position: Professor at University Medicine Göttingen and NCT & University Hospital Heidelberg, Scientific Director of TIGA Center for Medical Informatics / Bioinformatics / AI, Heidelberg
Area of expertise: AI based digital pathology, image analysis, medical informatics and bioinformatics
Email: niels.grabe(at)bioquant.uni-heidelberg.de
Chen Hong, PhD (DKFZ)
Position: Postdoc, Bioinformatician
Area of expertise: Translational Bioinformatics, Multi-omics Analysis, Bioinformatics Methods development
Email: c.hong(at)dkfz-heidelberg.de
Dimitrios Kalatzis, PhD (DKFZ)
Position: Postdoc
Area of expertise: Machine Learning, Explainable AI, Raman Spectroscopy
Email: dimitrios.kalatzis(at)nct-heidelberg.de
Ines Kühnel, PhD
Position: Senior Research Associate
Area of expertise: Immunology, Cellular therapy, CAR-T cells
Email: ines.kuehnel(at)nct-heidelberg.de
Marten Meyer, PhD (UKHD/DKFZ)
Position: Senior Research Associate, Deputy Head of the CCU Applied Tumor Immunity (DKFZ)
Area of expertise: translational research, TCR discovery, recombinant protein production
Email: marten.meyer(at)dkfz-heidelberg.de
Frank Momburg, PD MD (UKHD)
Position: Senior Research Associate
Area of expertise: translational research, TCR discovery, recombinant protein production
Email: frank.momburg(at)dkfz-heidelberg.de
Jordi Pfeifer Serrahima, PhD
Position: Postdoc
Area of expertise: Translational Research, Protein Engineering, TCR Discovery
Email: jordi.pfeiferserrahima(at)dkfz-heidelberg.de
Julia Pollmann, PhD
Position: Senior Research Associate
Area of expertise: Innate Immunity, Immuno-oncology, chronic viral infections
Email: j.pollmann(at)dkfz-heidelberg.de
Nevena Prodanovic (DKFZ)
Position: PhD student
Email: nevena.prodanovic(at)dkfz-heidelberg.de
Maria Paula Roberti, PhD (DKFZ)
Position: Senior Research Associate, Team Leader "Translational Research in Immuno-oncology and Microbiome" (TRIM)
Area of expertise: Immunotherapy, Translational Research, Gut microbiota and immunity
Email: mariapaula.roberti(at)nct-heidelberg.de
Alexander Rölle, PhD
Position: Senior Research Associate
Area of expertise: Innate Immunity, Cellular Immunology, Tumor Microenvironment
Email: a.roelle(at)nct-heidelberg.de
Patrick Schmidt, PhD (UKHD/DKFZ)
Position: Senior Research Associate, Team leader "Cellular therapies"
Area of expertise: Cellular therapy, CAR-T cells, TCR-T cells
Email: patrick.schmidt(at)nct-heidelberg.de
Svenja Schühle, PhD (DKFZ)
Position: Postdoc
Area of expertise: Tumor Microenvironment, Histology, Immunotherapy
Email: s.schuehle(at)dkfz-heidelberg.de
Silke Uhrig-Schmidt, PhD (DKFZ)
Position: Senior Research Associate
Area of expertise: AAV capsid design, cancer vaccination, gene and cell therapy
Email: silke.uhrig-schmidt(at)nct-heidelberg.de
Nektarios Valous, PhD (DKFZ)
Position: Senior Research Associate
Area of expertise: Computational image processing, computational biomedicine, interdisciplinary physics
Email: nek.valous(at)nct-heidelberg.de
Sergio Alberto Vasquez Urbina
Position: Research Associate, Bioinformatician
Area of expertise: Microbiome Metagenomics, Bioinformatics and Computational Systems Biology
Email: sergioalberto.vasquezurbina(at)dkfz-heidelberg.de
Katharina von Werthern (DKFZ)
Position: PhD Student
Email: katharina.vonwerthern(at)dkfz-heidelberg.de
Franziska Werner (DKFZ)
Position: PhD Student
Email: franziska.werner(at)dkfz-heidelberg.de
Jingyu Yang, PhD (DKFZ)
Position: Postdoc, Bioinformatician
Area of expertise: Explainable Artificial Intelligence (XAI), Graph Machine Learning, Bioinformatics Pipelines for Precision Medicine
Email: jingyu.yang(at)nct-heidelberg.de
Inka Zörnig, PhD
Position: Senior Research Associate, Project coordination and lab head
Area of expertise: translational research, biomarker discovery, immunotherapy
Email: inka.zoernig(at)nct-heidelberg.de
Technical Assistance
Martina Busacker-Scharpff
Isabella Gosch
Monika Hexel
Angela Holzer (DKFZ)
Iris Kaiser
Annette Köster
Alexandra Krauthoff (DKFZ)
Claudia Luckner-Minden
Sabrina Purr
Selina Seith (DKFZ/UKHD)
Claudia Tessmer (DKFZ)
Alexandra Tuch (DKFZ)
Claudia Ziegelmeier
Former Members
Dr. Aileen Berger
Dr. Julia Bitzer
Dr. Eren Boga
Dr. Sarah Glenz
Yanhong Lyu
Dr. Rodrigo Moraleda
Dr. Lasse Neukirch
Dr. Zeynep Koşaloğlu-Yalçın







