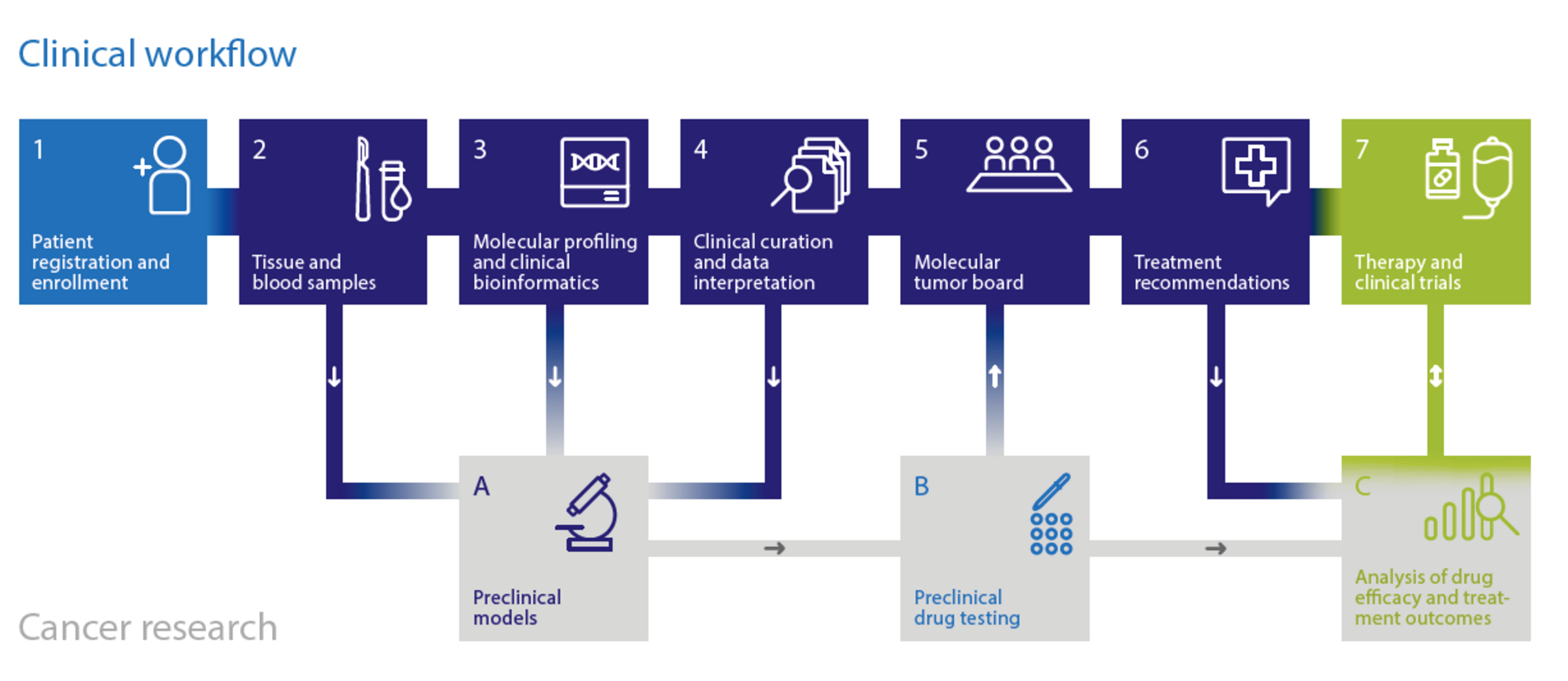
DKFZ/NCT/DKTK MASTER Workflow
“Precision oncology” describes the ability to predict which patients will likely respond to specific cancer therapies based on comprehensive, high-resolution molecular diagnostics as well as the functional understanding of individual tumors.
Such stratification of patients can be achieved, e.g., through next-generation sequencing of tumor DNA and RNA, revealing genomic alterations that have immediate clinical implications. DKFZ/NCT/DKTK MASTER provides a complete workflow for selection and consenting of patients, tissue processing, whole-exome/genome and RNA sequencing, bioinformatic analysis, and molecularly guided clinical decision making by molecular tumor boards, which are held three times a week and include members with expertise in clinical oncology, pathology, molecular biology, bioinformatics, and medical genetics and counseling.
Since many molecular alterations identified in human cancers have unknown functional consequences and can therefore not directly be interpreted regarding their suitability as therapeutic targets, separating “driver” mutations from biologically neutral “passenger” alterations is critical for translating genetic information into the clinic. Furthermore, the therapeutic value of known oncogenic mutations may vary depending on tissue context. To address these challenges, we investigate the functional role of genetic alterations predicted to be damaging in appropriate experimental systems, followed by the analysis of phenotypic consequences. The goal of these studies is to establish a versatile platform for rapid “functionalization” of individual molecular profiles and develop a continuously evolving, “learning” system to support treatment decisions at NCT.
Another priority is to constantly explore how technologies beyond whole-exome/genome and RNA sequencing can aid in patient stratification and individualization of treatment. Current efforts include the systematic evaluation of germline mutations affecting known cancer genes and the incorporation of additional layers of patient characterization, e.g., genome-wide DNA methylation profiling, various liquid biopsy approaches, targeted proteome analyses, and multiparameter magnetic resonance imaging.