NCT Tissue Bank
Despite considerable achievements in tumor treatment, there is a great demand for new therapeutic targets and for diagnostic markers and tests. An understanding of the molecular pathogenesis of the disease is a prerequisite for their systematic development.
Possibly the most important source of this knowledge is the examination of relevant disease-specific changes in human tumor tissue. During treatment or surgery, tissue is routinely obtained for therapeutic or diagnostic purposes and is examined in detail at the Heidelberg University Hospital’s Institute of Pathology.
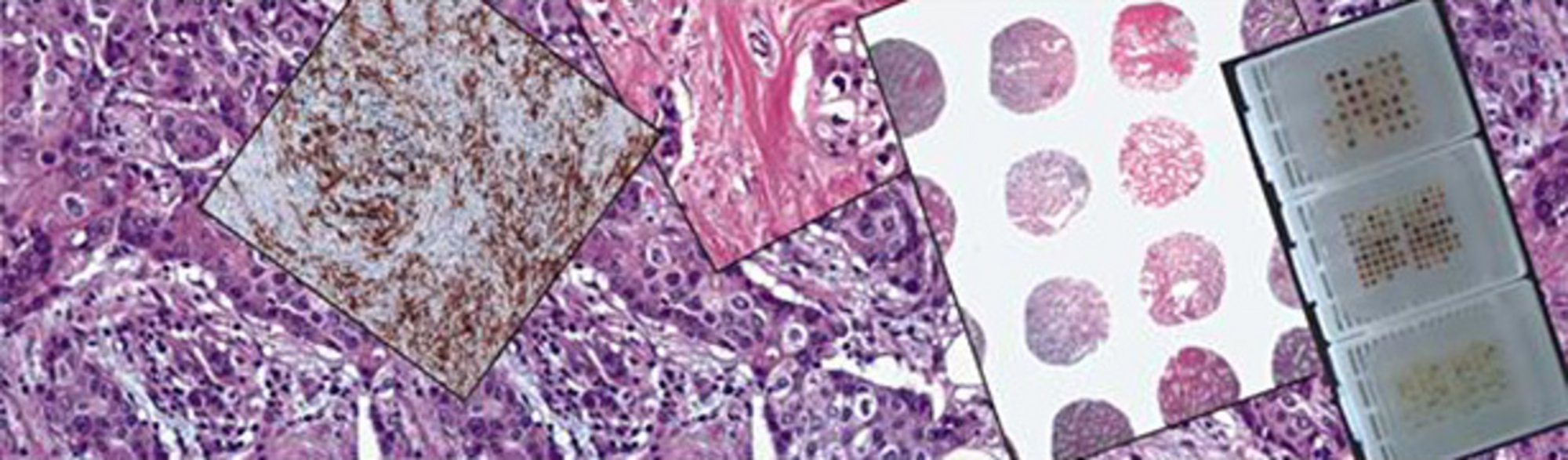
After all analyses and diagnostic procedures are completed, the NCT Tissue Bank collects the no longer needed remaining tissue and makes it available for research purposes in a formalin-fixed or shock-frozen state. This allows it to undergo further examination using the most modern methods. The objective is to continue to increase knowledge of tumor diseases and hence improve diagnostic and therapeutic measures.
The NCT Tissue Bank is an institution of the National Center for Tumor Diseases (NCT) Heidelberg under the patronage of the Medical Faculty of Heidelberg University and the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ). It is located in the Institute of Pathology of the Heidelberg University Hospital, by which it is supervised.
The mission of the NCT Tissue Bank is the collection, characterization, registration, archiving and processing of tissues and their derivatives (e.g. in extracts and tissue micro arrays) in high quality for scientific examination in tumor research.
Parallel to tissue collection, the NCT Tissue Bank also records the minimal basic data necessary for its work in a pseudonymized form.
Since the end of 2005, more than 10,000 tissue samples from a variety of tumors and corresponding, non-tumorous tissues have been preserved in a fresh state. The NCT Tissue Bank also has access to the extensive paraffin archive of the Heidelberg University Hospital’s Institute of Pathology and, through cooperation agreements, to extensive tissue collections of several national and international epidemiological studies.
The NCT Tissue Bank services offered through the Technologieplattform include the following:
- Standard and specialized histology techniques including paraffin and frozen section technology
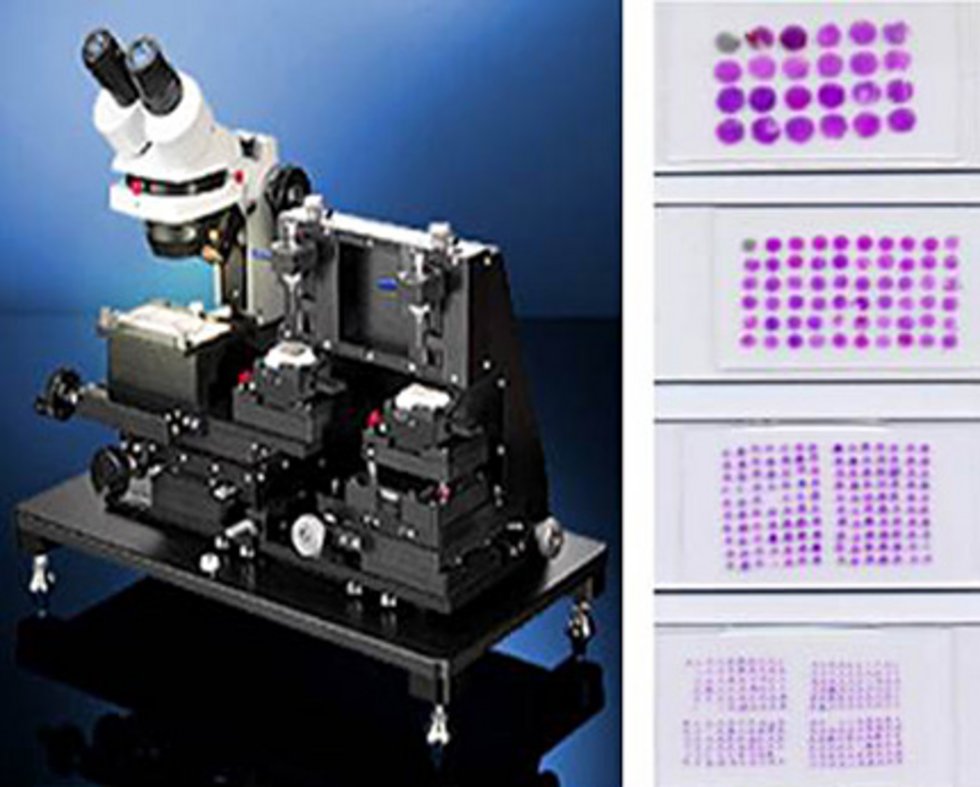
- Multi tissue arrays (tissue micro arrays)
These techniques allow the expression of disease-related gene products to be examined simultaneously in hundreds of tissue samples and new relevant markers or detection methods to be tested and assessed in terms of quality.
In the NCT Tissue Bank, histopathologically evaluated samples of especially common malignant tumor diseases (breast, lung, intestine, prostate, pancreas, kidney, liver, etc.) are used for the preparation of multi tissue arrays. At present (as of 01/2014) more than 90 entity-specific TMAs or screening TMAs (PAN tumor and PAN normal tissue arrays) with up to 100 corresponding tumor samples are available. - (DNA- and RNA) extraction procedures
- Immunohistology


- Under certain conditions there is an option for having standardized and fully automated immunohistological staining protocols for human tissue samples performed through the NCT Tissue Bank.
- Reference and training services
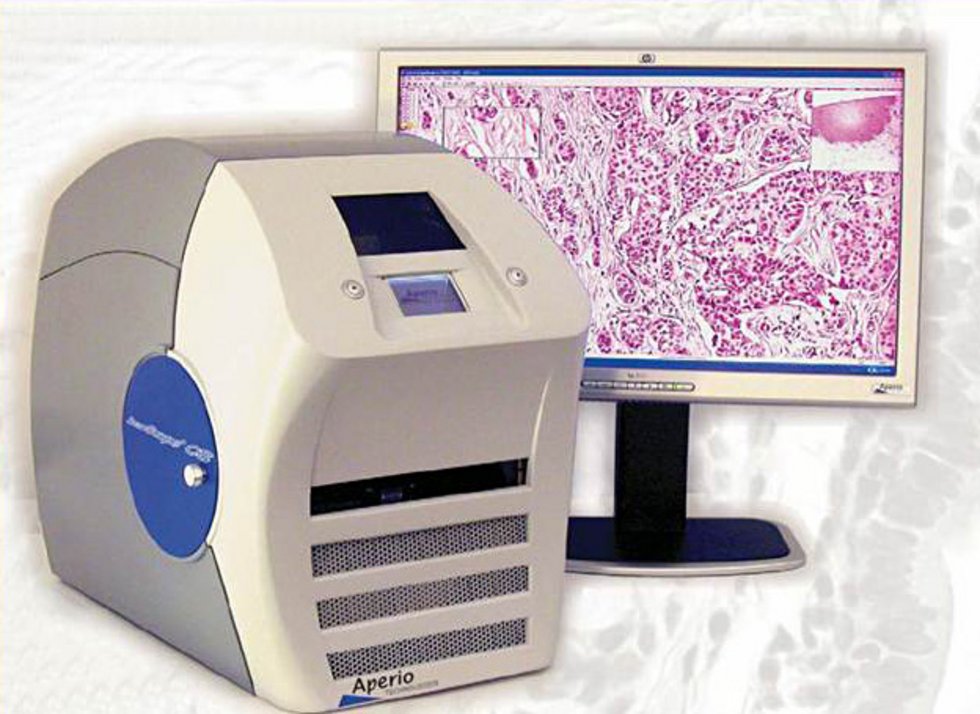
- Virtual microscopy
- Access to specialized technologies in cooperation (laser microdissection, electron microscopy, specialized microscopy techniques)
The NCT Tissue Bank supports biomedical scientific projects of the Medical Faculty, the Heidelberg University Hospital, the German Cancer Research Center and all NCT member institutions as well as other institutions contributing to the NCT Tissue Bank. Therefore employees of the institutions named above have the option of making use of tissue bank services upon request.
Each project requires a short (max. ½ page) project presentation by the project manager; the corresponding form may be downloaded from the Heidelberg University Hospital website.
Each project is promptly evaluated by the NCT Tissue Bank in consultation with the respective project manager as to feasibility and mode. Bilateral projects require the approval and competent supervision of the respective partners on the part of the University Hospital and Institute of Pathology; projects involving multiple clinical facilities, however, require approval by the Advisory Board of the NCT Tissue Bank. In the time period from 2005 to January 2014, the NCT Tissue Bank was able to support more than 1,300 projects. The proportion of approved and successfully completed projects is at present 98%.
The correct implementation and application of current data protection regulations and their legal and ethical basis is of great importance to the NCT Tissue Bank’s operations.
The management of both the Heidelberg University Hospital and the National Center for Tumor Diseases declare that the NCT Tissue Bank at the Institute of Pathology, in its function as an inspection body type C in line with DIN EN ISO/IEC 17020, is free from any commercial, financial or other influences. They assume responsibility that any exercise of influence by outside persons or organizations on services provided by the inspection body – examination findings and the provision of expertise – is excluded.
The positive ethics committee opinion concerning establishment of the tissue and tumor bank was issued by the Ethikkommission der Medizinischen Fakultät Heidelberg (ethics commission of the Heidelberg Medical Faculty) and is revalidated regularly.
The entire staff of the Tissue Bank are instructed on their obligation to maintain data confidentiality in line with §6 of the Landesdatenschutzgesetz (state law concerning data protection). Among other duties, they are instructed that it is prohibited without authorization to process, publish, use or make protected personal data available for any purpose other than the lawful and legitimate performance of duties, and that these obligations persist after termination of the duties.
The electronic storage and processing of data (morbidity data) complies with all data protection requirements. It is ensured that the data may not be traced to patients or that the linking of data to medical histories by third parties is not possible. The collected samples and data are stored or transmitted in a pseudonymized form, i.e. without names or identifying data. To this end, the tissue samples and personal data are encrypted by means of a numerical code. Mr. Martin Schurer is the University Hospital’s data protection officer.
Sophisticated tumor research or the application of research findings to improved therapeutic approaches is unthinkable without high quality and quality-controlled human tissue materials. Tissue banks therefore represent important resource and technology platforms that assume this role through the standardized collection, storage and processing of human tissues and their derivatives for research purposes.
Since 2006, the NCT Tissue Bank has operated a quality management system that is oriented towards the requirements of the DIN EN ISO/IEC 17020 standard, which includes the criteria stipulated by DIN EN ISO 9001. The NCT Tissue Bank has been accredited according to DIN EN ISO/IEC 17020 since 2009 and hence also orients itself towards “Good Scientific Practice” (GSP) criteria. In this context internal and external reviews of the quality management system are carried out at regular intervals.
Monitoring of the maintenance of accreditation is performed by the Deutsche Akkreditierungsstelle (DAkkS) (German Accreditation Body).
- Central biobanking structure, responsible for biobanking coordination and support of biomaterial-based projects in the Deutsches Konsortium für Translationale Krebsforschung (DKTK) (German Cancer Consortium)
- Central tissue bank structure of the National Cohort
- Competent biobanking institution of the epidemiological group projects for all tissue-specific questions:
ColoCare
DACHS
ESTHER
IMPACT
EPIC - Founding institute and coordination of tissue banking of the Arbeitsgemeinschaft der Onkologischen Spitzenzentren (AG CCC) (working group of the top oncological centers)
- Decisively involved in tissue banking of the Deutsches Zentrum für Lungenforschung (DZL) (German center for lung research)
Through its leading role in the AG Gewebebanking of the CCC and its coordinating function in biobanking at the German Health Centers and the epidemiological groups, the NCT Tissue Bank is closely networked with all tissue banks in Germany. Furthermore, the NCT Tissue Bank is integrated with the cBMB initiative of the TMF and an active member of the TMF and the German Biobank Node. International involvement includes links with the BBMRI and numerous bilateral cooperations with the University Hospital Zurich, Fudan University in Shanghai and the Infectious Diseases Biobank at Kings College in London.
The NCT Tissue Bank conducts extensive public relations work in order to inform tissue donors, users (scientists) as well as agencies, supporters and politically responsible institutions about goals and requirements of and services provided by tissue banking in general and about the NCT Tissue Bank and the institutions it supervises in particular.
This includes:
- The website
- Lectures and presentations at scientific symposia, meetings of interested parties, information events and other opportunities
- Publications on biobanking-related questions
At present, the NCT Tissue Bank services are provided at no cost to scientists in Heidelberg, with a few exceptions (industry partners, TMAs, IHC). The processing of complex matters may entail appropriate compensation.
The contact person for scientific questions and director of the NCT Tissue Bank is Dr. Alexander Brobeil.
Application forms
Application forms for tissue samples may be obtained via Downloads on the website of the NCT Tissue Bank.







