Institute for Medical and Data Ethics (IMDE) / Section Translational Medical Ethics
Previously: Ethics and Patient oriented Care in Oncology (EPOC)
Director: Prof. Dr. med. Dr. phil. Eva Winkler

Section Translational Medical Ethics (AG Winkler)
(f.l.t.r.): Dr. Aline Weis, Julie Schweer, Lukas Kiefer, Karla Alex, Dr. Katja Mehlis, Dr. Martin Jungkunz, Prof. Dr. Dr. Eva Winkler, Andreas Bruns, PhD, Isabell Haase-Saebel, Dr. Christoph Schickhardt, Dr. Julia König, PD Dr. Markus Herrmann, Lars Neth
Not in the picture: Andreas Wabro
The Section for Translational Medical Ethics is a dedicated core facility for applied ethics at the National Center for Tumor Diseases (NCT) Heidelberg, a partnership between German Cancer Research Center (DKFZ) and Heidelberg University Hospital, Medical Faculty, Heidelberg University. Main research areas are: Research Ethics (e.g. data driven & precision medicine, genome sequencing and editing), Clinical Ethics (e.g. end of life decisions, organizational ethics, AI ethics) and Public Health Ethics (e.g. resource allocation, secondary use of health data).
The section's expertise is represented in its participation in numerous initiatives and committees (e.g. Central Ethics Commission of the German Medical Association, Academy for Ethics in Medicine (AEM - Society for Medical Ethics in Germany), German Human Genome-Phenome Archive (GHGA), 1+mio Genomes Initiative, Genomic Data Infrastructure Project (GDI), Network of University Medicine (NUM), Medical Informatics Initiative (MII), EURAT platform (Ethical and Legal Aspects of Translational Medicine)).
Due to the impressing medical & technological progress fostered by structural and systemic changes in health care, ethical issues in oncology and research constantly gain importance. Such issues include, for instance, the informed consent process in research, patient’s rights and the right to self-determination or the ethical justification of expensive treatments facing limited resources in health care.
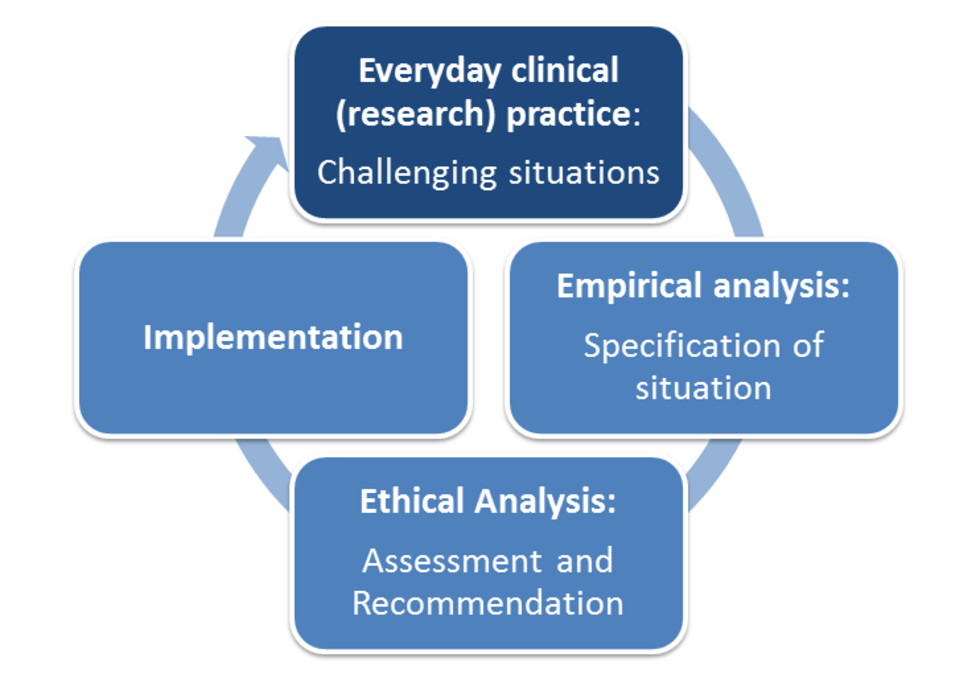
The concept of Empirical Ethics combines empirical studies in patient care and research with ethical analysis (cf. figure 1).
- The concept of empirical ethics starts with a clinical or research question that causes moral distress.
- In a first step, the empirical research allows for a detailed account of the ethical questions at stake.
- The ethical analysis weighs the values and ethical principles affected in order to develop an ethically sound recommendation.
- To close the concept, this recommendation aims at informing our decisions in the clinical or research context - be it as an aid in decision-making, as an advice or a counselling service.

Section Translational Medical Ethics research, teaching and clinical activities focus on:
Clinical Ethics
- End-of-Life Decision Making (EPAL)
- Ethical issues of the use of electronic health records (INFOPAT)
- Ethical issues in the care for elderly (PAC-E)
- Ethical guideline for advanced care planning in Oncology – adaptation and implementation (ELBA)
- HiGHmed – WP 7: Ethics and Stakeholders
- Preference-based decision aid to support participatory decisions about tumor-specific and palliative therapy in the last months of life (PETUPAL)
Research Ethics
- ITCC Hopp: International Data Integration Platform to prioritize drug development and access for children with cancer
- DAta FIDUciaries in MEdical Research: ethical and legal foundations and implementations (DAFIDUMER)
- Research Ethics and ethical requirements for the informed consent process in Next Generation Sequencing (EURAT)
- Big Data in Systems Medicine. Normative and Social Aspects for Physicians, Scientists, Patients and Society (DASYMED)
- CoNfirm – Subproject 3: Data Sharing and Data Protection
- Learning from Clinical Data. Ethical, Social and Legal Aspects (LinCDat)
- Comparative Assessment of Genome and Epigenome Editing in Medicine: Ethical, Legal and Social Implications (COMPASS-ELSI)
- FAIR-Data Spaces (FAIR-DS)
- German Human Genome-Phenome Archive (GHGA)
- Marsilius-Project: "Treuhand" (Fiduciary relationships in medical ethics)
Public Health Ethics
- Financial consequences of cancer in patients with neuroendocrine and colorectal tumors (Cancer and Poverty)
- Financial Effects of a Tumor Disease (FIAT)
- PREPARED: “PREparedness and PAndemic REsponse in Deutschland”
- Genomic NEWborn screening programs – Legal Implications, Value, Ethics and Society (NEW_LIVES)








