DISCOVER
Weiterführende Informationen sind nur auf Englisch verfügbar.
» Rational Modification of Oncolytic Viruses Using small RNAs
» Oncolytic Adenoviruses: Selecting and Engineering Adenovirus Serotypes with Improved Oncolytic and Immunostimulatory potency
» Mathematical Modeling of Combined Oncolytic Immunotherapy
» Heterotypic Spheroid Model Development for Preclinical Studies of Virotherapy-induced Changes of Tumor Microenvironment
» RadioViroTherapy for Treatment of Refractory Solid Cancer
» Viro-antibody therapy: Engineering oncolytic adenoviruses for genetic delivery of therapeutic antibodies
» Boosting oncolytic viruses by small molecule compounds
» RNA switch-inducible genetic delivery of biotherapeutics by oncolytic viruses
» Oncolytic vaccination therapy for treatment of solid cancer
» Exploiting chimeric oncogenic transcription factors to target therapeutic viruses to pediatric tumors
Rational Modification of Oncolytic Viruses Using small RNAs
PI (s)
- Mathias F. Leber
Contributing CCU members
- Adrian Eilert, Katia Dittus, Marie Szczeponik, Jessica Albert, Birgit Hoyler, Stefanie Sawall
- Scientific staff (alumni): Sophie Anker, Jan Dessila, Hans-Martin Singh, Marc-Andrea Bärtsch, Sascha Bossow
Virotherapy is a novel treatment modality that employs tumor-specific, oncolytic viruses (OVs), which directly lyse cancer cells and induce an anti-tumor immune response. Small RNAs are a broad class of endogenous or exogenous nucleic acids that are defined by their limited size (<200 nt) and their non-coding nature (i.e., they act directly as an RNA molecule and do not encode proteins). Small RNAs have broad implications in numerous areas including control of endogenous transcripts, innate immunity and defense against viral pathogens, as well as biotechnology and biomedical research.
In this project, we explore multiple ways to rationally modify and thereby improve OVs using small RNA-based systems and technologies.
Sub-project: “Control of OV replication by genomic insertion of synthetic microRNA target sequences for enhanced safety”: Restriction of OV replication to tumor cells is paramount for safety when administering OVs to cancer patients. Therefore, we have developed and optimized a microRNA-based post-entry restriction system that blocks oncolytic measles virus replication in healthy cells. In this approach, synthetic target sequences for microRNAs, which are lost during tumorigenesis, but are present in healthy cells, are incorporated into the viral genome. Thereby, virus replication in healthy cells is suppressed by the host cell’s RNAi-machinery while replication in and destruction of tumor cells is unhindered.
Sub-project: “Boosting OV efficacy by encoding therapeutic small RNAs”: Here, we are developing genetically engineered viruses which encode small RNAs for targeted knock-down of host factors restricting OV replication specifically in tumor cells. Thereby, we aim to increase the oncolytic potency and therapeutic efficacy of OVs.
Ultimately, we aim to develop these rationally modified OVs into clinically administered therapeutics for the benefit of cancer patients.
Publications
- Oncolytic Measles Virus Encoding MicroRNA for Targeted RNA Interference. Anker, S.C.; Szczeponik, M.G.; Dessila, J.; Dittus, K.; Engeland, C.E.; Jäger, D.; Ungerechts, G.; Leber, M.F. Viruses 2023 Jan 22;15(2):308
- MicroRNA-sensitive oncolytic measles virus for chemovirotherapy of pancreatic cancer. Singh HM, Leber MF, Bossow S, Engeland CE, Dessila J, Grossardt C, Zaoui K, Bell JC, Jäger D, von Kalle C, Ungerechts G. Molecular Therapy Oncolytics 2021 May 5;21:340-355.
- Engineering and combining oncolytic measles virus for cancer therapy. Leber MF, Neault S, Jirovec E, Barkley R, Said A, Bell JC, Ungerechts G. Cytokine & Growth Factor Reviews 2020 Jul 3:S1359-6101(20)30149-0.
- Enhanced Control of Oncolytic Measles Virus Using MicroRNA Target Sites. Leber MF, Baertsch MA, Anker SC, Henkel L, Singh HM, Bossow S, Engeland CE, Barkley R, Hoyler B, Albert J, Springfeld C, Jäger D, von Kalle C, Ungerechts G. Molecular Therapy Oncolytics 2018 Apr 12;9:30-40.
- MicroRNA-mediated multi-tissue detargeting of oncolytic measles virus. Baertsch MA, Leber MF, Bossow S, Singh M, Engeland CE, Albert J, Grossardt C, Jäger D, von Kalle C, Ungerechts G. Cancer Gene Therapy 2014 Sep;21(9):373-80.
- MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Leber MF, Bossow S, Leonard VH, Zaoui K, Grossardt C, Frenzke M, Miest T, Sawall S, Cattaneo R, von Kalle C, Ungerechts G. Molecular Therapy2011 Jun;19(6):1097-106.
Funding
- Physician-Scientist Fellowship to MFL, Heidelberg University, Medical Faculty
Oncolytic Adenoviruses: Selecting and Engineering Adenovirus Serotypes with Improved Oncolytic and Immunostimulatory Potency
PI(s)
- Dirk M. Nettelbeck
Contributing CCU members
- Jonny Hertzog, Levi Antonius Kuon, Annemarie Stotz, Katja Schaudin, Jessica Genz, Stefanie Sawall
Cooperation partners
- Claudia Ball, National Center for Tumor Diseases, Dresden and German Cancer Research Center, Heidelberg, Germany
- Anja Ehrhardt and Wenli Zhang, University of Witten-Herdecke, Germany
- Benedikt Brors and Sadaf Shabbir Mughal, German Cancer Research Center, Heidelberg, Germany
- Niels Halama, National Center for Tumor Diseases, Heidelberg; University Hospital Heidelberg and German Cancer Research Center, Heidelberg, Germany
Oncolytic adenoviruses possess many advantageous features for applications in virotherapy, including unique mechanisms of tumor-selectivity, immunostimulatory activity supporting oncolytic tumor vaccination and various engineering opportunities for adapting or improving oncolytic functions. However, previous work has focused on one of now >100 known adenovirus serotypes.
The aim of this program is to characterize the oncolytic and immunological properties of adenovirus serotypes and engineer lead candidates towards a new generation of efficiency-enhanced oncolytic adenoviruses. To this end, we systematically screen recently established adenovirus libraries (cooperation with Anja Ehrhardt, Univ. of Witten-Herdecke) in tumor and immune cell cultures and in sophisticated patient-derived pre-clinical tumor models (cooperation with our colleagues at NCT and DKFZ, Claudia Ball and Niels Halama). Comprehensive analyses include various virological and immunological parameters at the molecular and cellular level and in the context of tumor tissue.

Funding
- Wilhelm Sander-Stiftung, project grant (to DMN)
- DKFZ International Postdoc Program
- German Cancer Aid (Fellowship to LAK)
Mathematical Modeling of Combined Oncolytic Immunotherapy
PI(s)
- Christine E. Engeland, Johannes P. W. Heidbüchel
Contributing CCU members
- Jessica Albert, Birgit Hoyler, Stefanie Sawall
Cooperation partners
- Daniel Abate-Daga and Dr. Heiko Enderling, Moffitt Cancer Center, Tampa, FL, USA
- Patrick Schmidt and Dirk Jäger, National Center for Tumor Diseases, University Hospital Heidelberg and German Cancer Research Center Heidelberg
- Richard Harbottle, German Cancer Research Center Heidelberg
- Michael Breckwoldt, University Hospital Heidelberg and German Cancer Research Center Heidelberg
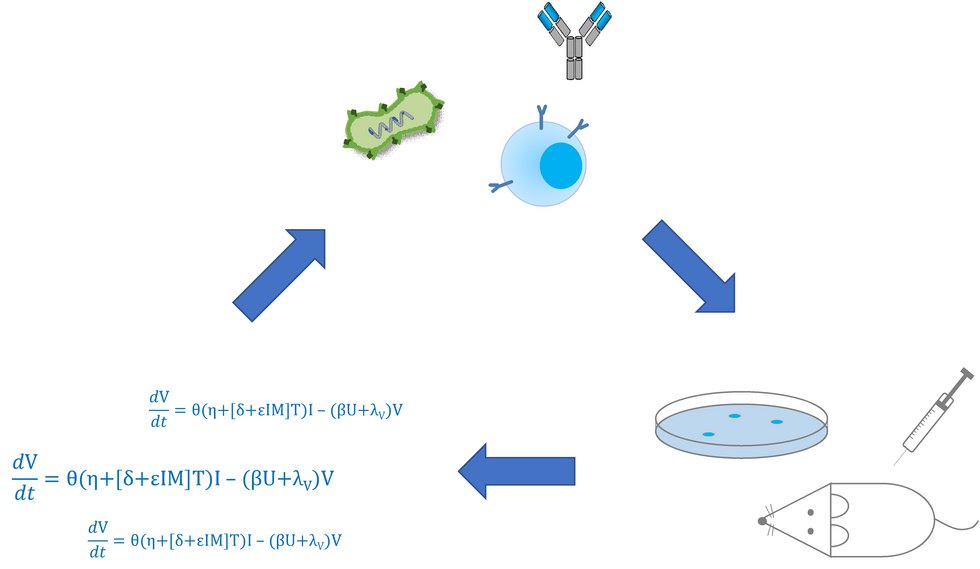
Cancer immunotherapies, such as immune checkpoint blockade and chimeric antigen receptor (CAR) T cell therapy, have recently proven successful in numerous pre-clinical and clinical studies. However, critical challenges including toxicities and lack of efficacy, especially against solid tumors, have yet precluded benefit for the majority of patients. Combination with oncolytic viruses which directly lyse tumor cells and stimulate an anti-tumor immune response has been shown to be a viable option to increase the therapeutic window of immunotherapy. However, the complexity of the immune system and the plethora of possible combinations rule out comprehensive testing to identify optimal treatment schedules.
To address these challenges, we are developing mathematical models of combination immunovirotherapies together with our collaborators from Moffitt Cancer Center in Tampa, FL, Dr. Daniel Abate-Daga and Dr. Heiko Enderling.
Lines of research include parametrization of the model with quantitative experimental data. In interdisciplinary collaborations, we are working together with Jessica Hunger and Dr. Dr. Michael Breckwoldt (DKFZ) on imaging approaches and with Aileen Berger, Dr. Patrick Schmidt, Prof. Dr. Dirk Jäger (University Hospital Heidelberg), Alice de Roia, Dr. Matthias Bozza and Prof. Dr. Richard Harbottle (DKFZ) on CAR design and development.
Following validation and refinement, the models will be used to simulate outcomes of theoretical combination therapies in order to prioritize the most promising treatment approaches for further development. Crucial parameters in the system will be identified as targets for therapeutic intervention. This innovative workflow will accelerate clinical translation of combination immunotherapies for the benefit of cancer patients.
Publications
- Mathematical modeling of oncolytic virotherapy. Heidbuechel JPW, Abata-Daga D, Engeland CE, Enderling H. In: Engeland CE (ed.) Oncolytic Viruses. Methods in Molecular Biology vol 2058, Humana, New York, NY, ISBN 978-1-4939-9793-0.
- Targeted BiTE Expression by an Oncolytic Vector Augments Therapeutic Efficacy Against Solid Tumors. Speck T*, Heidbuechel JPW*, Veinalde R, Jaeger D, von Kalle C, Ball CR, Ungerechts G, Engeland CE. Clinical Cancer Research 2018; 24: 2128-37.
- Fighting cancer with mathematics and viruses. Santiago DN*, Heidbuechel JPW*, Kandell WM, Walker R, Djeu J, Engeland CE, Abate-Daga D, Enderling H. Viruses 2017; 9(9), 239.
Funding
- Wilhelm Sander Foundation, Grant No. 2018.058.1 (to CEE), NCT Heidelberg School of Oncology (HSO²), Postdoctoral Fellowship in Clinical Cancer Research (to JPWH)
Heterotypic Spheroid Model Development for Preclinical Studies of Virotherapy-induced Changes of Tumor Microenvironment
PI(s)
- Assia Angelova
Contributing CCU members
- Jean Rommelaere (Prof. Emeritus), Alexandra Just, Milena Barf
Rodent protoparvoviruses possess natural tumor-killing (oncolytic) properties. Furthermore, these viruses, H-1PV in particular, are capable of reversing tumor-driven immune suppression through the induction of immunogenic tumor cell death and the establishment of a proinflammatory tumor microenvironment.
H-1PV immunostimulating effects have been demonstrated both preclinically and in early phase clinical trials: ParvOryx01 (glioblastoma) (https://clinicaltrials.gov/ct2/show/NCT01301430) and ParvOryx02 (pancreatic cancer) (https://clinicaltrials.gov/ct2/show/NCT02653313).
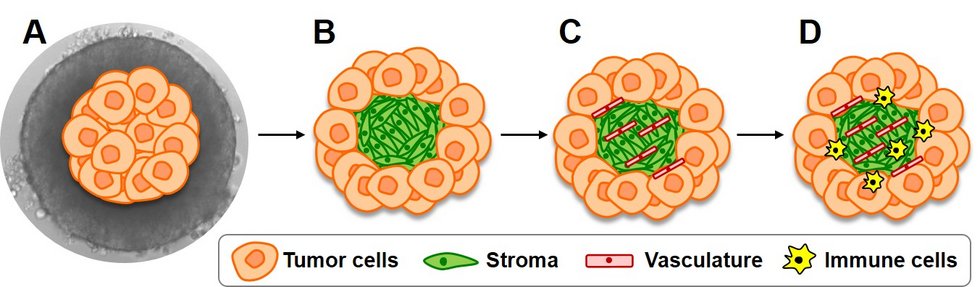
Parvovirotherapy has potential as anticancer strategy and the prospects of development of parvovirus-based combinatorial treatments urge the need for relevant preclinical models applicable to both basic and translational viro-immunotherapy research. The main goal of our team is to establish in vitro systems, which mimic the structural and functional complexity of the tumor microenvironment, including the interplay between stroma, vasculature and immune cells. We apply a methylcellulose-based hanging drop approach to generate double, triple and quadruple heterotypic spheroids consisting of tumor, fibroblast, endothelial and immune cells. These complex 3D systems may be subjected to various viro-immunotherapeutic treatments and further implemented into transwell co-culture, organotypic or animal models.
We are currently focused on pancreatic cancer spheroid model establishment. We are also pursuing the reproducible generation of non-Hodgkin lymphoma (NHL) spheroids, taking up the challenge of the intrinsically low 3D growth capacity of blood cancer cells. Diffuse large B cell lymphoma and cutaneous T cell lymphoma, two NHL subtypes presently lacking pertinent tumor microenvironment recapitulation in vitro, are in the spotlight of our research activities. The ultimate goal of our team is the establishment of a translationally relevant, patient tumor material-based heterotypic spheroid model as a novel platform for preclinical viro-immunotherapeutic testing.
In collaboration with K. Geletneky (Darmstadt, Germany), B. Leuchs (DKFZ), J. Nüesch (DKFZ), A. Marchini (DKFZ and LIH, Luxembourg).
Publications
- Fluorescence In Situ Hybridization (FISH) Detection of Viral Nucleic Acids in Oncolytic H-1 Parvovirus-Treated Human Brain Tumors. Kiprianova I, Just A, Leuchs B, Rommelaere J, Angelova AL. Methods Mol Biol 2020; 2058: 295-306.
- Immune Conversion of Tumor Microenvironment by Oncolytic Viruses: The Protoparvovirus H-1PV Case Study. Marchini A, Daeffler L, Pozdeev VI, Angelova A, Rommelaere J. Front Immunol 2019; 10: 1848.
- Immune System Stimulation by Oncolytic Rodent Protoparvoviruses. Angelova A, Rommelaere J. Viruses 2019; 11: 415.
- Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Geletneky K, Hajda J, Angelova AL, Leuchs B, ...Just A, ....Rommelaere J. Mol Ther 2017, 25: 2620-34.
- The Oncolytic Virotherapy Era in Cancer Management: Prospects of Applying H-1 Parvovirus to Treat Blood and Solid Cancers. Angelova AL, Witzens-Harig M, Galabov AS, Rommelaere J. Front Oncol 2017; 7: 93.
RadioViroTherapy for Treatment of Refractory Solid Cancer
PI(s)
- Mathias F. Leber, Guy Ungerechts
Contributing CCU members
- Katia Dittus, Assia Angelova, Jessica Genz, Birgit Hoyler, Stefanie Sawall
Cooperation Partners
- Laurent Daeffler, Institut Pluridisciplinaire Hubert Curien, CNRS/University of Strasbourg, France
- Karim Plath, Department of Otorhinolaryngology, Head and Neck Surgery, Heidelberg University Hospital, Heidelberg, German
- Claudia Ball, National Center for Tumor Diseases, Dresden and German Cancer Research Center, Heidelberg, Germany
- Richard Harbottle, German Cancer Research Center Heidelberg, Germany
- Rienk Offringa, German Cancer Research Center, Heidelberg, Germany
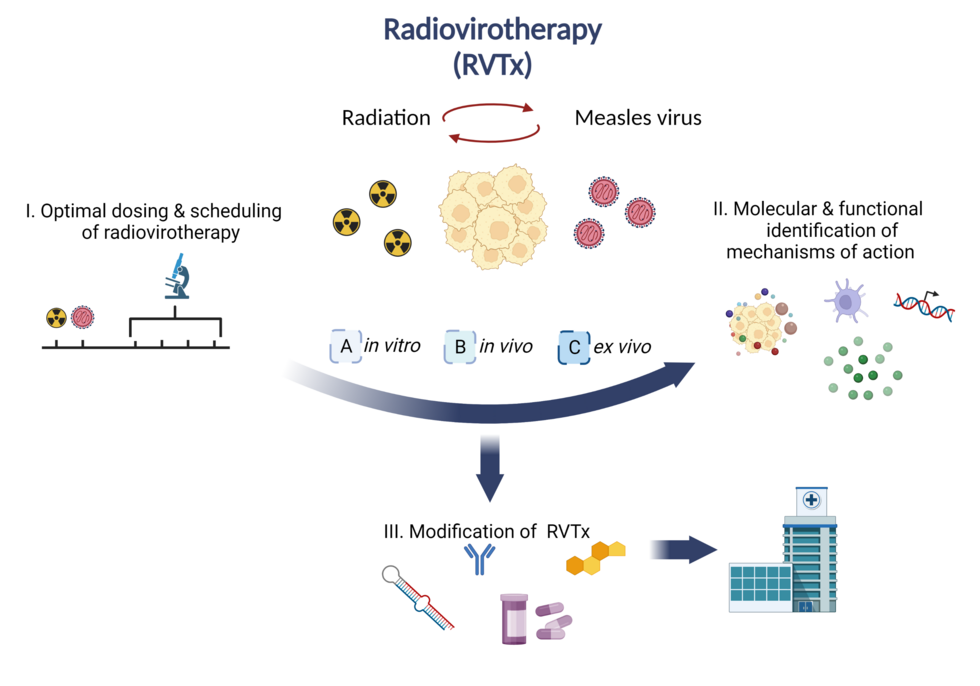
Refractory tumors such as pancreatic ductal adenocarcinoma (PDAC), head and neck squamous cell carcinoma (HNSCC) or glioblastoma (GBM) remain a major challenge in medical oncology. To address the limited clinical efficacy of current standard therapies, we aim to investigate virotherapy using oncolytic measles virus (MeV) or parvovirus (PV) combined with radiotherapy. We hypothesize that combined radiovirotherapy (RVTx) may elicit synergistic anti-cancer effects and induce a sustained anti-tumor immune response.
We are analyzing different dosing schedules of radiation and oncolytic MeV and have confirmed synergy of RVTx in HNSCC and PDAC cell lines in vitro. An in-depth molecular and functional characterization revealed elevated levels of immunogenic cell death (ICD) in response to RVTx, measured by classical ICD markers. To investigate therapeutic effects of RVTx in models of high translational relevance, we employ various ex vivo models such as patient-derived tumor explant samples or spheroids/organoids. We aim at elucidating susceptibility to viral infection as well as decipher innate and adaptive immune responses upon RVTx. Recently, we expanded our radiation platform to include particle radiation. In the future, the molecular and functional characterization as well as immunophenotyping will allow for a rational modification of RVTx in order to develop combination virotherapy regimens for improved treatment efficacy.
Funding
- Alois Hirdt-Erben und Wieland-Stiftung Heidelberg
- Bi-national ANR-DFG grant: Combination of proton radiations and oncolytic viruses to eradicate cancer – PROTOVEC; Agence nationale de la recherche & German Research Foundation.
Viro-antibody therapy: Engineering oncolytic adenoviruses for genetic delivery of therapeutic antibodies
PI
- Dirk M. Nettelbeck
Contributing CCU members
- Martin Boos, Jessica Albert, Stefanie Sawall
Cooperation partners
- Roland Kontermann and Oliver Seifert, University of Stuttgart, Germany
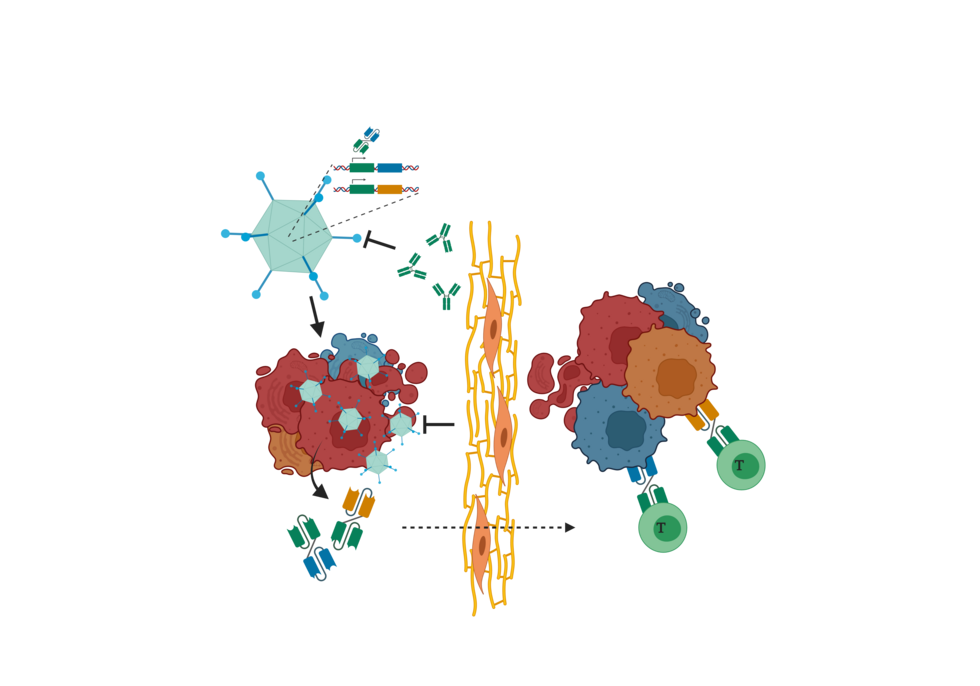
Oncolytic viruses specifically infect tumor cells followed by virus replication and spread in the tumor, thereby lysing the infected tumor cells. These features also recommend oncolytic viruses for targeted and self-amplifying delivery of therapeutic proteins to tumors by inserting the corresponding genes into the viruses’ genomes. As an example, oncolytic viruses facilitate local and prolonged expression of therapeutic antibodies in tumors (viro-antibody therapy, Kontermann et al., 2021). This approach might facilitate superior tumor perfusion compared with standard antibody treatment and combine viral oncolysis with bystander killing of non-infected tumor cells.
The aim of this program is to engineer oncolytic adenoviruses for the genetic delivery of small recombinant antibody therapeutics targeting a set of tumor antigens. In a collaboration with Roland Kontermann of the University of Stuttgart, we explore different recombinant antibody formats and antibody-mediated effector mechanisms for their suitability for viro-antibody therapy. Moreover, we engineer oncolytic adenoviruses to investigate and optimize different expression strategies for potent and tumor-specific (co-)expression of antibodies. For the resulting antibody-delivering oncolytic adenoviruses, virus- and antibody-mediated activities as well as combined therapeutic efficacy are analyzed in cell cultures and tumor models in vitro and in vivo.
These developed oncolytic virus platforms will allow for the switching of antibody specificities and/or effector domains to facilitate broader applications in cancer therapy.
Publications
- Kontermann RE, Ungerechts G, Nettelbeck DM. Viro-antibody therapy: engineering oncolytic viruses for genetic delivery of diverse antibody-based biotherapeutics. mAbs 2021;13: 1982447
- Fernandez-Ulibarri I, Hammer K, Arndt MA, Kaufmann JK, Dorer D, Engelhardt S, Kontermann RE, Hess J, Allgayer H, Krauss J, Nettelbeck DM. Genetic delivery of an immunoRNase by an oncolytic adenovirus enhances anticancer activity. Int J Cancer 2015;136: 2228-40.
Funding
- Studienstiftung des Deutschen Volkes (German National Academic Foundation)
Boosting oncolytic viruses by small molecule compounds
PI(s)
- Mathias F. Leber, Dirk Nettelbeck, Guy Ungerechts
Contributing CCU members
- Marie Szczeponik, Juliane Hastedt, Adrian Eilert, Birgit Hoyler, Jessica Genz, Stefanie Sawall
Cooperation partners
- Tommy Alain, Children’s Hospital of Eastern Ontario (CHEO), Research Institute I, Ottawa, ON, Canada
- Karim Plath, Department of Otorhinolaryngology, Head and Neck Surgery, Heidelberg University Hospital, Heidelberg, Germany
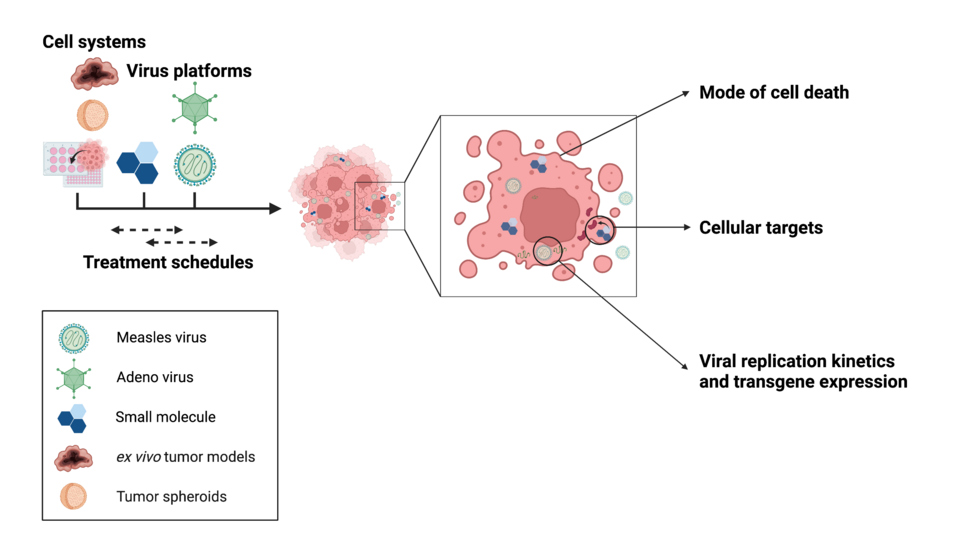
In recent years, oncolytic viruses have emerged as a promising therapy modality for solid and recurrent tumors. However, their ability to elicit durable remissions in patients is limited. In this project we aim at combining oncolytic measles and adenoviruses with small molecule compounds (pharmacovirotherapy, PVTx) to overcome limitations to virotherapy and achieve a better therapeutic efficacy.
Small molecules are a class of therapeutic drugs that can specifically target pathways and counteract resistance factors in the host cells and can therefore act as an optimal partner for virotherapy.
Together with our cooperation partners, we aim to screen small molecules in their ability to boost the viruses’ cytolytic activity, performing several viability assays. We will be further investigating the compounds’ effect on viral replication and transgene expression. In a next step, we will perform in-depth analyses of the mechanisms underlying potential synergism. Among other aspects, we will be looking at the mode of cell death, as well as potential target proteins in the host cells, e.g. by knocking down specific targets using siRNAs.
Finally, we will be assessing the therapeutic efficacy in preclinical tumor models with the objective of eventually translating our results into the clinic.
Funding
- Deutsche Krebshilfe (Mildred-Scheel-Doktorandenprogramm)
RNA switch-inducible genetic delivery of biotherapeutics by oncolytic viruses
PI
- Dirk M. Nettelbeck
Contributing CCU members
- Laura Kayser, Pierre Gommeringer, Jessica Albert, Stefanie Sawall
Cooperation partner
- Jörg Hartig, University of Konstanz, Germany
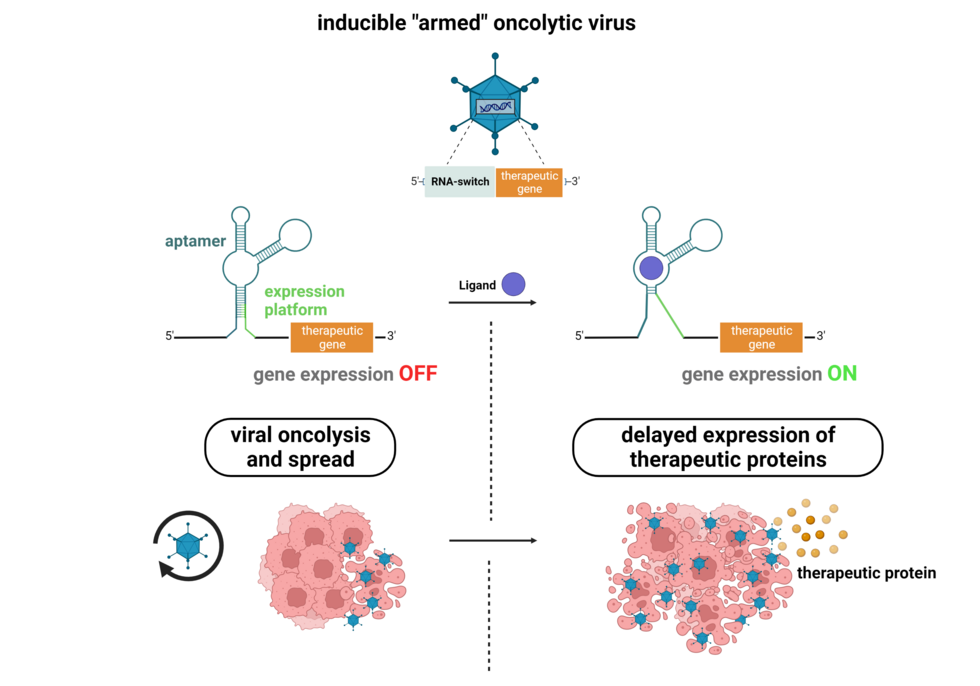
Most oncolytic viruses, including adenoviruses, provide the unique opportunity to express therapeutic proteins in tumors for improved therapeutic outcome. As such, the genetic delivery of biotherapeutics, including apoptosis-inducing ligands, fusion proteins, cytokines, or recombinant antibodies is under investigation. However, this approach may be challenged by interference with oncolytic virus replication or toxic side effects. The aim of our work is to develop, in close collaboration with Jörg Hartig’s group at the University of Konstanz, RNA switches for ligand-dependent regulation of oncolytic virus-delivered therapeutic genes, in order to facilitate optimal timing or as safety switch-off.
In previous collaborative work, we have shown proof-of-principle for RNA switch-regulated expression both of oncolytic virus-mediated transgene expression and of oncolytic virus replication (DNA and RNA viruses) using a model aptazyme, which is a RNA sequence combining a ligand-binding aptamer and a self-cleaving ribozyme.
In on-going work, we generate and analyze medically applicable eukaryotic RNA switch designs, optimize their performance, and investigate functional insertion sites in oncolytic virus genomes. Ultimately, we will investigate different approaches for enhancing therapeutic potency of oncolytic viruses without compromising their safety by RNA switch-inducible expression of therapeutic payloads.
Publications
- Ketzer P, Haas SF, Engelhardt S, Hartig JS, Nettelbeck DM. Synthetic riboswitches for external regulation of genes transferred by replication-deficient and oncolytic adenoviruses. Nucleic acids research 2012;40: e167.
- Ketzer P, Kaufmann JK, Engelhardt S, Bossow S, von Kalle C, Hartig JS, Ungerechts G, Nettelbeck DM. Artificial riboswitches for gene expression and replication control of DNA and RNA viruses. Proceedings of the National Academy of Sciences of the United States of America 2014;111: E554-62.
Funding
- German Cancer Aid (Deutsche Krebshilfe), project grant
Oncolytic vaccination therapy for treatment of solid cancer
PI(s)
- Mathias F. Leber, Guy Ungerechts
Contributing CCU members
- Juliane K. Hastedt, Birgit Hoyler, Jessica Genz, Stefanie Sawall
Cooperation Partners
- Angelika Riemer, German Cancer Research Center, Heidelberg, Germany
The idea of administering therapeutic cancer vaccines has been around for several decades. It is hypothesized that the immunogenic context of the tumor antigen delivery induces a T-cell anti-tumor response leading to long-term immunity. Although clinical trials involving a variety of cancer vaccine designs have been conducted, the successful translation into routine oncology has been slow. A major challenge seems to be the lacking immunogenicity of previously developed vaccines given the various immunosuppressive mechanisms employed by cancer cells.
In this project, we aim at developing an oncolytic virus-based therapeutic cancer vaccine for treatment of solid cancer. Further details can be provided upon request.
Funding
- Deutsche Krebshilfe, Mildred Scheel Fellowship
Exploiting chimeric oncogenic transcription factors to target therapeutic viruses to pediatric tumors
PI(s)
- Dirk M. Nettelbeck with cooperation partner Thomas G. P. Grünewald
Contributing CCU members
- Eleonora Prodi
Cooperation Partners
- Thomas G. P. Grünewald, Division of Translational Pediatric Sarcoma Research, German Cancer Research Center (DKFZ), Heidelberg, Germany
The outcome of children, adolescents, and young adults with high-risk sarcomas, such as Ewing sarcoma (EwS) and alveolar rhabdomyosarcoma (ARMS), is still unacceptably low. These tumors (and other aggressive cancers) are fueled by and addicted to chimeric oncogenic transcription factors (OTFs) generated by chromosomal translocations that fuse otherwise unrelated DNA-binding and transactivating domains. Due to their tumor-specific expression, OTFs are in principle ideal therapeutic targets, but thus far, all attempts to completely suppress their activity have been unsuccessful which is why they are often classified as being undruggable. In collaboration with Thomas Grünewald’s lab, we challenge this dogma with a novel strategy to leverage, rather than to suppress, OTFs by exploiting their exquisite neomorphic DNA-binding preferences: Strikingly, in both EwS and ARMS, the respective OTFs can bind to completely different DNA sequences than their wildtype constituting TFs, thus conveying outstanding tumor-selective transcription control.
Therapeutic viruses have been developed for treatment of cancer by tumor-restricted viral infection, replication, cell lysis and spread. Such virotherapies critically depend on the restriction of virus replication to tumor cells. Based on own pioneering work (Hölting et al. 2022), this project exploits neomorphic OTF-binding regulatory DNA elements to restrict the expression of essential viral genes and therapeutic payloads to EwS, ARMS and other tumors. We plan to genetically engineer the viral genomes and to analyze their tumor-restricted activation and therapeutic gene expression and resulting therapeutic activity in tumor cell lines and established pre-clinical tumor models.
The resulting therapeutic viruses are candidates for further development toward clinical application in the translational program of our unit. Furthermore, applications of the OTF-mediated targeting strategy to other tumors and other therapeutic viruses is envisioned.
Publications
- Hölting TLB, Cidre-Aranaz F, Matzek D, Popper B, Jacobi SJ, Funk CM, Geyer FH, Li J, Piseddu I, Cadilha BL, Ledderose S, Zwilling J, Ohmura S, Anz D, Künkele A, Klauschen F, Grünewald TGP*, Knott MML*. Neomorphic DNA-binding enables tumor-specific therapeutic gene expression in fusion-addicted childhood sarcoma. Mol Cancer 2022;21(1):199.
- Kontermann RE, Ungerechts G, Nettelbeck DM. Viro-antibody therapy: engineering oncolytic viruses for genetic delivery of diverse antibody-based biotherapeutics. mAbs 2021;13: 1982447.
- Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev 2009; 61(7-8):554-71.
Funding
- European Union







