NCT Cancer Registry
The role of NCT Cancer Registry to document clinical occurring data in regard of a tumor patient started in Heidelberg in 2007 with the achievement of completeness of all cancer cases since 2008. In the light of digitalization and continuous development of medical knowledge and methods tumor documentation gained in complexity. The NCT Cancer Registry is responsible to document each cancer case within the NCT, whether being a primary patient, a patient with recurrent disease to be treated in an advanced stage and even a patient demanding a second opinion. Medical tumor documentation includes diagnosis and therapy information as well as staging information and information about the course of the disease. Approximately 13.000-14.000 new cancer patients are registered annually. Of these, about 30 percent are demands on second opinion, with tendency to rise.
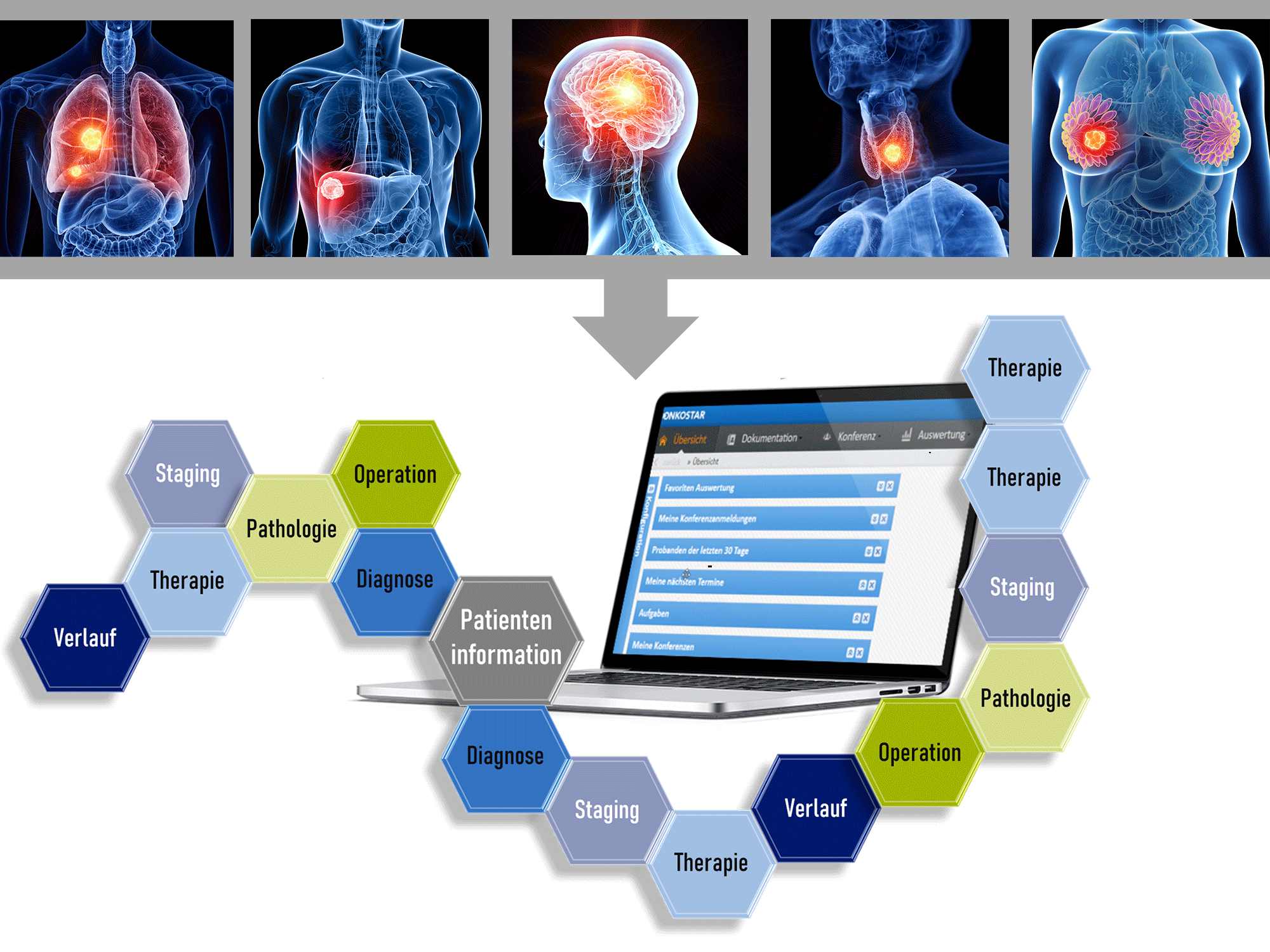
Demographic change is a perceptible factor of the last decades also apparent from annually increasing amounts of newly recorded tumor patients. The combination of massive loads of data and rapid scientific progress in the medical field (e.g. new developed standard techniques such as molecular diagnosis or immunohistochemistry) which are already daily routine have the desired effect to recognize the malignancy and head-on appropriate therapy. Standardized patient-centered medical care is closer than ever.
A prerequisite to use this big load of medical data for quick usage in the clinics and further research is, however, the obligation for the uniform application of medical documentation. As NCT Cancer Registry, we are working on a way to unify medical cancer documentation and thereby hosting state-of-the-art documentation of all cancer patients for upcoming new therapies and better patient care. By creating the standardized medical tumor documentation system (tumor documentation software Onkostar by IT-Choice, Karlsruhe), the NCT Cancer Registry focusses on bringing big data into line for optimizing evaluation and quality control. The focus lies on the well-arranged documentation of the step-by-step therapy progress including all examinations and follow-up therapies carried out after patient diagnosis.
In order to be able to compare and evaluate the data from the NCT cancer registry, they are encrypted according to national and international regulations. This set of rules has been compiled in entity-specific manuals, giving insight into the way the data collection is stored. Furthermore, they contain recommendations for encryption, rules for documentation and tumor classifications for specific organs, anatomically confined organ areas and organ systems:
- code ICD-O-3 histology according to current WHO classification
- ICD-O-3-localization
- ICD-10-diagnosis
- regional lymph nodes
- metastases
- multiple tumors
- tumor-specific staging
- classification
- etc.

The NCT clinical cancer registry - as an important interdisciplinary entity - pursues several objectives:
1. Obligation of the periodical reporting of all tumor diseases (C and certain D diagnoses) to the Landeskrebsregister Baden-Württemberg, which is mandatory since 2009

Data provision and analysis of registration data in an appropriate format and, on agreement, also as targeted, sophisticated analysis:
- for transnational research projects
- for clinical/epidemiological research projects
- for internal quality assurance
- for the prioritization of future NCT work
3. The clinical cancer registry serves as the basis for reports for funding institutions, e.g. Deutsche Krebshilfe, and certifying bodies
4. Certification of oncological centers at the NCT (e.g. detailed documentation and key data extraction) and quality assurance of oncologic treatment is supported by the NCT cancer registry
5. the registry also conducts independent clinical/epidemiological research- and method-oriented development projects
6. Training Services for medical documentalists
Projects
Head: Dr. rer. nat. Antje Brockschmidt
Research Associate: B. Sc. Sophia Schäfer
In the framework of the governmental program Medical Informatics Initiative, the BMBF (German Federal Ministry of Education and Research) is funding the consortium HIGHmed which consists of the three specialized use cases: oncology, cardiology and infection control. These three Use Cases entail the concept of a long-term vision of virtual platforms to simplify the rapid communication of data-based state-of-the-art research results and direct decision-making processes in the clinics for the purpose of tailored patient care.
The HIGHmed Use Case Oncology fostered by the NCT Cancer Registry Heidelberg focalizes upon the inter-site incorporation of huge data volumes (omics data) received from molecular analyses, genome sequencing and radiology. The idea of close interaction and information networking between university hospitals, research institutes, doctors and patients via direct visualization in a virtual oncology center is set up to reveal similar cancer cases with interoperable data integration solutions and methods to adjust to patient therapy accordingly.
» The HiGHmed Use Case Oncology | HiGHmed
» HiGHmed | Medizininformatik-Initiative (medizininformatik-initiative.de)
Contact person NCT Cancer Registry: Dr. rer. nat. Antje Brockschmidt
Research Associate: B. Sc. Sophia Schäfer
The Hopp Children's Cancer Center Heidelberg (KiTZ) is a joint institution of the German Cancer Research Center (DKFZ), the Heidelberg University Hospital (UKHD) and the Heidelberg University (UniHD). It is a unique institution that enables the research results of childhood tumors in Heidelberg to be implemented more quickly and more effectively in precise therapies.
To better supply patients with oncological and hematological diseases, the Society of Paediatric Oncology and Haematology (GPOH) created platforms to collect data on the disease itself, potential treatment options, as well as complications, and provides it for scientific analysis.
ZIPO registry is hosted by NCTR cancer registry.
» KiTZ Clinical Trial Unit
Contact person NCT cancer registry: Dr. rer. nat Beate Köper
Contact person Med. Onko V: Prof. Dr. med. Sascha Dietrich, Dr. med. Nora Liebers
SMARTTrial registry is hostet by NCT cancer registry.
» Clinical trials
» Universitätsklinikum Heidelberg: Prof. Dr. med. Sascha Dietrich
Contact person: Dr. rer. nat. Antje Brockschmidt
Research Associate: B. Sc. Sophia Schäfer
Registry of Liver Cancer Heidelberg is hostet by NCT cancer registry.
» LCCH – Liver Cancer Center Heidelberg
Contact person: Dr. rer. nat. Antje Brockschmidt
Research Associate: B. Sc. Sophia Schäfer
ZPM registry is hosted by the NCT cancer registry.
Collaborations
- MIRO – Medizinische Informatik in der Radioonkologie Heidelberg
- IT Choice, Karlsruhe
- LKR – Landeskrebsregister Baden-Württemberg
- ATO – Arbeitsgemeinschaft der Tumorzentren, Onkologischen Schwerpunkten und Arbeitskreis in Baden-Württemberg
- CCC Netzwerk – Netzwerk der onkologischen Spitzenzentren der Deutschen Krebshilfe
- ADT – Arbeitsgemeinschaft deutscher Tumorzentren e.V.
- DVMD – Fachverband für Dokumentation und Informationsmanagement in der Medizin
- GEKID – Gesellschaft der epidemiologischen Krebsregister in Deutschland







