Clinical trials – Gynaecological oncology
Clinical studies are essential to investigate and further develop the effectiveness and safety of new therapies. They also give patients access to innovative diagnostic and treatment methods that are not yet available outside of clinical trials. The study center of the Department of Gynecological Oncology at NCT Heidelberg has many years of experience in the planning and implementation of clinical studies for breast cancer and gynecological malignancies.
Diagnostic and therapeutic trials in early breast cancer: COGNITION and COGNITION-GUIDE
COGNITION and COGNITION-GUIDE
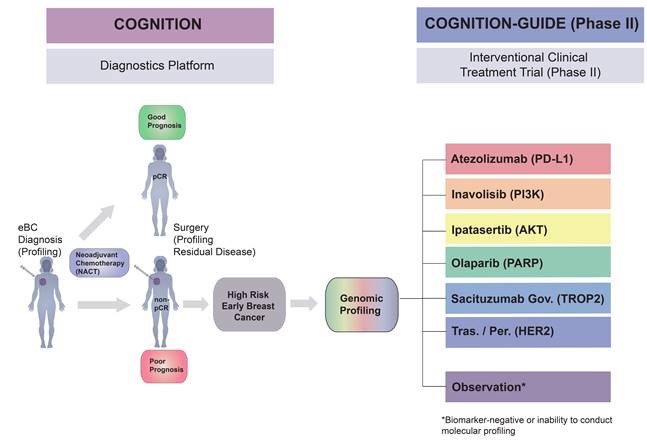
The COGNITION trial is aimed at patients with early stage (non-metastatic) breast cancer who are being treated with neoadjuvant therapy. As part of the COGNITION study, a genetic analysis of the tumor takes place to identify alterations that can be targeted. Ideally, study enrollment occurs prior to initiation of neoadjuvant chemotherapy, but is still possible if there is a poor response to chemotherapy prior to surgery.
The affiliated treatment trial COGNITION-GUIDE will investigate whether additional therapy guided by the individual genetic profile can further improve treatment outcome and prevent recurrence in breast cancer patients at high risk. This targeted therapy starts after completion of subsequent standard therapy and lasts 12 months. The targeted therapy depends on the results from the molecular analysis performed in the COGNITION. Enrolment in the COGNITION-GUIDE trial usually occurs after standard therapy, which is recommended following surgery.
1. COGNITION: You may be considered for participation if you have been diagnosed with early stage breast cancer and are planned to be treated with neoadjuvant therapy, i.e. therapy prior to surgery. In case of a poor response to neoadjuvant therapy, trial enrolment is also possible during neoadjuvant therapy/ before surgery.
2. COGNITION-GUIDE: A basic requirement for participation in the COGNITION-GUIDE trial is a genetic analysis of the tumor within the COGNITION diagnostic program. If you are eligible for the COGNITION-GUIDE trial, based on the risk of tumor recurrence, we will discuss this with you at the NCT.
How do I register?
If you would like a treatment at the NCT, you are welcome to send us your treatment documents by post. We use your documents to check whether we can offer you participation in a clinical trial at the NCT. If we think you might benefit from a clinical trial at our facility, we will contact you.
» Further information in the CATCH/COGNITION flyer (PDF file)
Appointment
Tel.: +49 6221 56-7985
Fax: +49 6221 56-5320
cognition.nct@med.uni-heidelberg.de
Frauenklinik.Ambulanz.Onkologie@med.uni-heidelberg.de
Patientenzentrum2.NCT@med.uni-heidelberg.de
Address
Patientenzentrum 2
Im Neuenheimer Feld 460
69120 Heidelberg
Please note the information under „Frequent questions“ and information provided by the Cancer Information Service (KID) about clinical trials (only available in German).
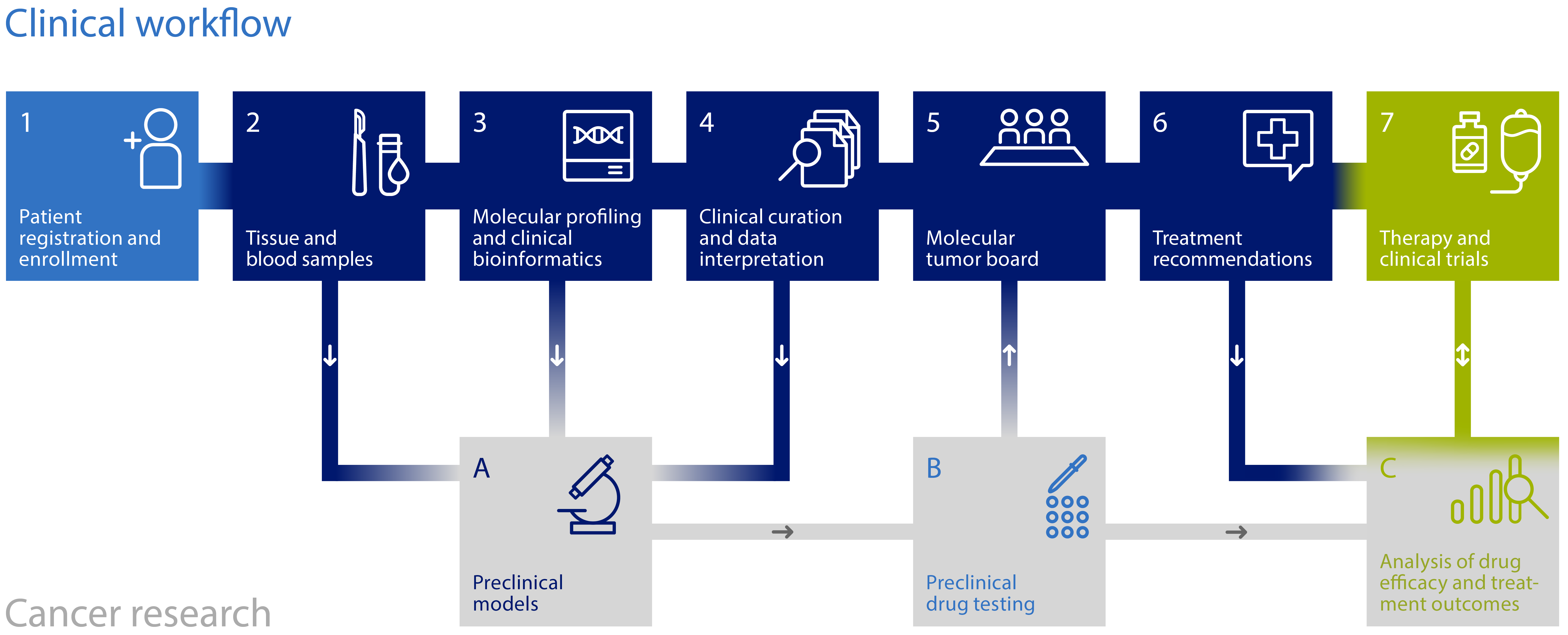
Trial inclusion for COGNITION and COGNITION-GUIDE, the detailed information about the studies, the planned diagnostics and, in the case of COGNITION-GUIDE, the offered therapy, are carried out by the treating physicians at the NCT Heidelberg. In the further course, the studies will take place at other NCT sites in Germany. The following information should help you to clarify initial questions.
Why do we analyze the genetic material of a tumor?
Cancer is caused by pathological changes in the genome. The pattern of these changes can differ significantly between people and between different tumors. The identification of individually modified genetic patterns helps to better understand each individual disease and possibly treat it in a targeted manner. The targeted use and combination of suitable drugs that are adapted to molecular targets should further improve the treatment of cancer and prevent patients from experiencing unnecessary side effects.
What is targeted therapy?
Currently, increasing amounts of medications, that specifically target altered molecules in malignant cells, are approved. These medications influence genetically modified cancer cells preferably and mostly spare healthy cells of the body. So-called small molecules or antibodies are examples for such medications, which inhibit growth of malignant cells through targeted attack within a tailor-made molecular therapy.
What does the abbreviation COGNITION stand for?
COGNITION stands for “Comprehensive assessment of clinical features, genomics and further molecular markers to identify patients with early cancer for enrolment on marker driven trials“.
What do we need?
For trial enrolment, we need a current medical report, as well as current examination findings. On the basis of these documents, we will check whether the trials are suitbale for you.
You will be informed in detail about the study and the planned diagnostics during an appointment at the gynecological-oncology outpatient clinic at the NCT Heidelberg (later also at other NCT sites). In order to be able to carry out the molecular tests, we need your written consent. In addition, we need fresh tumor tissue and a blood sample.
What will be done with the samples of the COGNITION trial?
First, the tumor tissue sample is pathologically assessed to determine the tumor cell content. This is an important indicator to decide whether extended molecular diagnostics (tumor genome sequencing) is feasible and evaluable. We then extract DNA, the carrier of genetic information, and RNA, the construction plan of important molecules of the cell, from your tumor material. Sequencing is then used to determine and compare the genetic codes of the tumor and healthy tissue. In addition, the blood is analyzed for inherited mutations.
What are the results of the analysis?
If your material is suitable for sequencing, we create a profile of tumor-specific changes. By comparing the sequence of the tumor tissue with that of the healthy tissue, we obtain information about changes in the tumor. Only some of the alterations found are known targets for approved drugs. For a large proportion of the alterations, bioinformatic analyses, expert knowledge and additional tests in the laboratory have to be combined in order to evaluate chances of success of a targeted therapy. Therapeutic options and the underlying data are discussed by medical and scientific experts in an interdisciplinary molecular tumor board.
How will I be notified that results are available?
The molecular tumor board decision will be communicated to you by your treating physician at the NCT. At this time, you will receive information about which treatment options are available in the affiliated clinical trial (COGNITION-GUIDE). For participation in the COGNITION-GUIDE trial, we again require your written consent.
Which therapy is offered in the COGNITION-GUIDE trial?
In the COGNITION-GUIDE clinical trial, 6 different drugs are available as therapy. Some of the investigational drugs (IMPs) are already approved by the authorities for the treatment of breast cancer and / or for other cancers (atezolizumab, olaparib, sacituzumab govitecan, trastuzumab/pertuzumab), some are still in clinical trials (inavolisib, ipatasertib). With the exception of olaparib and trastuzumab/pertuzumab, the drugs have not yet been approved specifically for the treatment of early breast cancer.
Will I always receive an investigational drug in COGNITION GUIDE?
Which study arm you are assigned to and which investigational drug you will receive depends on the detected molecular aberrations in your tumor tissue. If no molecular changes are detected that allow assignment to a treatment arm, or if analysis of the tissue material is not possible, you will be included in an observational arm of the study.
When does therapy start in COGNITION-GUIDE?
The targeted therapy in the COGNITION-GUIDE trial will begin 1-3 months after standard therapy ends. The type and duration of the standard therapy depends on the breast cancer subtype. The expected duration of treatment with the investigational drug in COGNITION-GUIDE is 12 months.
Who coordinates COGNITION and COGNITION-GUIDE?
Trial conducted in close collaboration between the German Cancer Research Center (DKFZ) and Heidelberg University Hospital (UKHD) as well as the National Center for Tumor Diseases (NCT) Heidelberg.
Overview of Recruiting Centers
COGNITION
Heidelberg
National Center for Tumor Diseases (NCT) Heidelberg
Prof. Dr. med. Andreas Schneeweiss
Berlin
Charité - Universitätsmedizin Berlin, Department of Gynaecology incl. Breast Cancer CCM/CBF
Prof. Dr. med. Jens-Uwe Blohmer
Erlangen
Friedrich-Alexander-Universität Erlangen-Nürnberg, University Hospital Erlangen, Womens Hospital
Prof. Dr. med. Peter Fasching
Ulm
University Hospital Ulm, Womens Hospital
Prof. Dr. med. Wolfgang Janni
Planned Recruitment COGNITION
Dresden
University of Technology Dresden, Medical Faculty and University Hospital Carl Gustav Carus
Prof. Dr. med. Pauline Wimberger
Tübingen
University Hospital Tübingen
Prof. Dr. Andreas Hartkopf
Würzburg
Womens Hospital and Polyclinic of the University Hospital
Prof. Dr. med. Achim Wöckel
Regensburg
Clinic for Gynecology and Obstetrics of the University of Regensburg at Caritas Hospital St. Josef
PD Dr. med. Stephan Seitz
Augsburg
University Hospital Augsburg
Prof. Dr. Nina Ditsch
Essen
University Hospital Essen, Clinic for Gynecology and Obstetrics
PD Dr. med. Oliver Hoffmann, PD Dr. med. Anne-Kathrin Bitter
Köln
University Hospital Cologne, Breast Center Cologne
Dr. med Wolfram Malter
COGNITION GUIDE
Heidelberg
National Center for Tumor Diseases (NCT) Heidelberg
Prof. Dr. med. Andreas Schneeweiss
Berlin
Charité - Universitätsmedizin Berlin, Department of Gynaecology incl. Breast Cancer CCM/CBF
Prof. Dr. med. Jens-Uwe Blohmer
Erlangen
Friedrich-Alexander-Universität Erlangen-Nürnberg, University Hospital Erlangen, Womens Hospital
Prof. Dr. med. Peter Fasching
Planned Recruitment COGNITION GUIDE
Dresden
University of Technology Dresden, Medical Faculty and University Hospital Carl Gustav Carus
Prof. Dr. med. Pauline Wimberger
Ulm
University Hospital Ulm, Womens Hospital
Prof. Dr. med. Wolfgang Janni
Tübingen
University Hospital Tübingen
Prof. Dr. Andreas Hartkopf
Würzburg
Womens Hospital and Polyclinic of the University Hospital
Prof. Dr. med. Achim Wöckel
Augsburg
University Hospital Augsburg
Prof. Dr. Nina Ditsch
Essen
University Hospital Essen, Clinic for Gynecology and Obstetrics
PD Dr. med. Oliver Hoffmann, PD Dr. med. Anne-Kathrin Bitter
Clinical trial CATCH
The clinical trial CATCH is aimed at patients with advanced breast cancer (metastasis). The aim of the trial is to offer further individual targeted therapy options that are tailored to individual genetic tumor biology in addition to standard procedures. Patients must be treated at the NCT Heidelberg from the date of enrolment into the study.
Conditions of participation
Participation can be considered if you are diagnosed with advanced stage / metastatic breast cancer and it is possible to acquire tissue for molecular diagnostics during the standard routine bioptic or surgical procedures.
How do I register?
If you would like a treatment at the NCT, you are welcome to send us your treatment documents by post. We use your documents to check whether we can offer you participation in a clinical trial at NCT. Due to the current Covid situation, please understand the longer processing times. If we think you might benefit from a clinical trial at our facility, we will contact you.
» Further information in the CATCH/COGNITION flyer (PDF file)
Appointment
Tel.: 06221 56-7985
Fax: 06221 56-5320
Frauenklinik.Ambulanz.Onkologie@med.uni-heidelberg.de
Patientenzentrum2.NCT@med.uni-heidelberg.de
Address
Patientenzentrum 2
Im Neuenheimer Feld 460
69120 Heidelberg
Please note the information under „Frequent questions“ and information provided by the Cancer Information Service (KID) about clinical trials (only available in German).
Treating physicians at NCT Heidelberg will screen for enrolment criteria (inclusion into the trial) and clarify all details of the trial and planned diagnostics. The following information is intended to help you answer the first questions.
Why is the genetic material of a tumour analysed?
Cancer is caused by abnormal changes in the genome. The pattern of these changes can vary widely between people and between different tumors. The identification of individually modified genetic patterns helps to better understand each individual disease and possibly treat it in a targeted manner.
Prospectively, the targeted use and combination of suitable drugs that are adapted to molecular target structures should further improve the treatment of cancer and prevent patients from experiencing unnecessary side effects of ineffective medication.
What is targeted therapy?
Currently, increasing amounts of medications, that specifically target altered molecules in malignant cells, appear on the market. These medications influence genetically modified cancer cells preferably and mostly spare healthy cells of the body. So-called small molecules or antibodies are examples for such medications, which inhibit growth of malignant cells through targeted attack within a tailor-made molecular therapy.
»Targeted therapy: How does it work? (ePaper KID, only available in German)
What does the abbreviation CATCH stand for?
CATCH stands for “Comprehensive assessment of clinical features and biomarkers to identify patients with advanced or metastatic breast cancer for marker driven trials in humans“.
What do we need?
A current physician's letter and all results of current medical examinations is required to check eligibility for trial enrolment. By examining the latter, we will then check whether your participation is appropriate to the current status of the disease.
During an appointment as part of the consultation of the gynecological-oncological outpatient clinic at the NCT Heidelberg, you will be informed in detail about the clinical study and the planned diagnosis. In order to be able to carry out the molecular tests, we need your written consent. We also need fresh tumor tissue and a blood sample for comparison. If immediate intervention in the routine standard is planned, please contact us in advance to synchronize processes.
What will be done with the samples of the CATCH trial?
First, the tumor tissue sample is examined pathologically to determine the tumor cell content. This is an important indicator for deciding whether the extended molecular diagnostics (tumor genome sequencing) can also be conducted and evaluated. We then extract DNA, the carrier of hereditary information, and RNA, the construction plan of molecules in a cell from the tumor material. The genetic codes of tumor and healthy tissue are determined and compared by sequencing.
Which results are delivered by the analysis?
By comparing the sequences of tumorous and healthy tissue, information about changes in the tumor can be obtained. Only a small proportion of the differences identified are known target structures for validated medication. For a large part of the differences, bioinformatic analyses, expert knowledge and additional tests in the laboratory have to be combined in order to evaluate potential routes of targeted therapies. Medical and scientific experts in an interdisciplinary molecular tumor panel discuss specific therapy options and underlying data.
How do I get to know about results?
Your treating physician at the NCT Heidelberg / a participating study center will provide you with advice from the Molecular Tumor Board for a potential further targeted therapy within an affiliated advanced clinical trial.
Do I definitely get therapy advice?
If your material is suitable for sequencing, we will create a profile of tumor-specific changes. In this case the tumor profile does not match with any existing therapeutic options, we cannot suggest new therapy options. If there are important points in the tumor profile for personalized therapy, the treating oncologist at NCT Heidelberg / a participating study center will inform you. Possible options are treatment with a drug that is approved for the therapy of other diseases (off-label use), but also appears promising for this special form of breast cancer but also drugs, which are approved for breast cancer (in-label use). In addition, there is the possibility of being included in a clinical trial if the corresponding targets are available.
Who coordinates CATCH?
Trial conducted in close collaboration between the German Cancer Research Center (DKFZ) and Heidelberg University Hospital (UKHD) as well as the National Center for Tumor Diseases (NCT) Heidelberg.
Overview of Recruiting Centers
CATCH
Heidelberg
National Center for Tumor Diseases (NCT) Heidelberg
Prof. Dr. med. Andreas Schneeweiss
Berlin
Charité - Universitätsmedizin Berlin, Department of Gynaecology incl. Breast Cancer CCM/CBF
Prof. Dr. med. Jens-Uwe Blohmer
Augsburg
University Hospital Augsburg
Prof. Dr. Nina Ditsch
Planned Recruitment CATCH
Dresden
University of Technology Dresden, Medical Faculty and University Hospital Carl Gustav Carus
Prof. Dr. med. Pauline Wimberger
Erlangen
Friedrich-Alexander-Universität Erlangen-Nürnberg, University Hospital Erlangen, Womens Hospital
Prof. Dr. med. Peter Fasching
Ulm
University Hospital Ulm, Womens Hospital
Prof. Dr. med. Wolfgang Janni
Tübingen
University Hospital Tübingen
Prof. Dr. Andreas Hartkopf
Würzburg
Womens Hospital and Polyclinic of the University Hospital
Prof. Dr. med. Achim Wöckel
Regensburg
Clinic for Gynecology and Obstetrics of the University of Regensburg at Caritas Hospital St. Josef
PD Dr. med. Stephan Seitz
Essen
University Hospital Essen, Department of Internal Medicine (Tumor Research), Hematology and Oncology
PD Dr. med. Anja Welt
Köln
University Hospital Cologne, Breast Center Cologne
Dr. med Wolfram Malter
Further Clinical Trials
The Division of Gynecological Oncology conducts numerous further diagnostic, treatment, observational and clinical trials.
»You can find an overview under "Clinical trials".