Molecular Precision Oncology
Current molecular stratification programs & clinical trials
Molecular Precision Oncology Program
- MASTER - Molecularly Aided Stratification for Tumor Eradication
- CATCH - Comprehensive Assessment of Clinical Features and Biomarkers To Identify Patients with Advanced or Metastatic Breast Cancer for Marker Driven Trials in Humans
- COGNITION - Comprehensive assessment of clinical features, genomics and further molecular marker to identify patients with early breast cancer for enrolment on marker driven trials
- NCT Neuro Master Match (N2M2)
- INdividualized Therapy FOr Relapsed Malignancies in Childhood (INFORM)
- Personalizing Refractory Myeloma Therapy (PeRMyT)
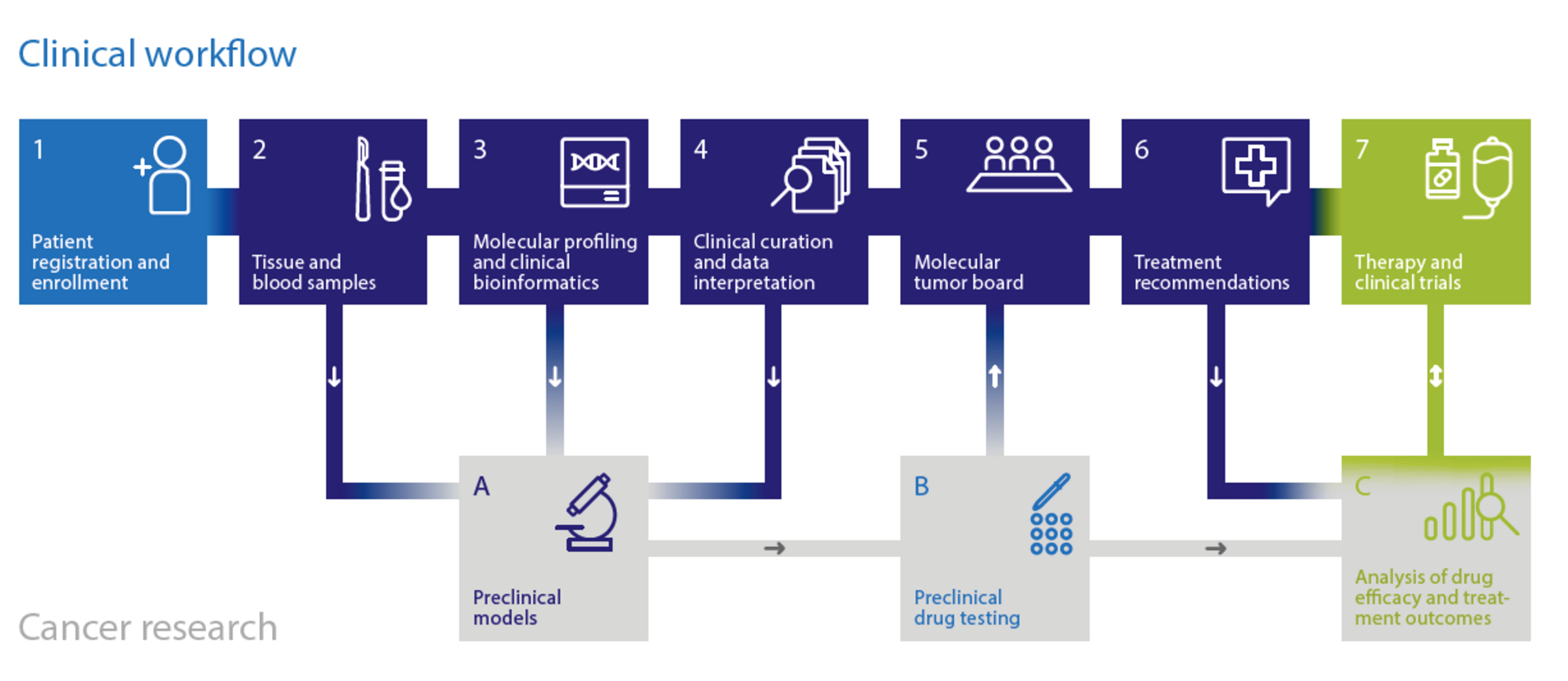
A central goal of the NCT is molecularly guided patient stratification as the basis for individualized treatment decisions in somatic and hereditary cancers.
By leveraging the expertise of NCT, DKFZ, and DKTK in multidimensional tumor characterization and molecular mechanism-based therapy, NCT has developed a standard for a) comprehensive molecular profiling, b) clinical interpretation of molecular data, c) functional analysis of primary patient samples, d) treatment decision making in molecular tumor boards, e) longitudinal clinical data collection and f) clinical trial design and conduct. The current workflow is being expanded by additional layers of patient characterization, such as genome-wide DNA methylation profiling, which has substantially advanced the diagnosis and classification of brain tumors and sarcomas. To improve clinical translation, a portfolio of molecularly guided clinical trials has been developed, and the biological stratification approach will be extended to additional treatment modalities.
Current molecular stratification programs include clinical studies, such as the NCT MASTER program, which is a registry for young adults with advanced cancer across histologies and adults with rare tumors across age groups. The personalized oncology registry trials CATCH and COGNITION, which focus on metastatic breast cancer (CATCH) and early-stage breast cancer (COGNITION). N2M2, an umbrella trial for patients with newly diagnosed MGMT-promoter-unmethylated glioblastoma. The INFORM registry study, which enrolls all children and adolescents with relapsed or refractory cancers in Germany and 12 other countries for comprehensive molecular profiling. The Personalizing Refractory Myeloma Therapy (PeRMyT) program, which is a program for biomarkers-based studies for patients with multiple myeloma.
All of these clinically applicable, diagnostic platforms provide whole-exome/genome and RNA sequencing when standard treatment has failed and routine molecular diagnostics have yielded no actionable target.
Potentially actionable findings are discussed in molecular tumor boards, including participants from the NCT Heidelberg, the NCT Dresden, and all DKTK (German Cancer Consortium) partner sites, to determine therapeutic choices for individual patients. Towards the goal of systematic clinical translation, the molecular profiling platforms are linked to a range of investigator initiated trials (IITs), such as the NCT PMO (Personalized Medical Oncology) studies (e.g.TOP-ART), which are coordinated by the Clinical Trial Center at NCT Heidelberg and Soratram Protocol or Basket of Baskets (BoB).