Sarcomas are rare mesenchymal tumors that can arise from connective and supporting tissue, muscle, bone and cartilage and can occur anywhere in the body. They are characterized by a great histological diversity and can have a very variable clinical course. While some tumor forms can grow aggressively and form metastases, others show a less severe development. Although certain sarcomas occur more frequently at a young age, patients of all ages are affected. All these circumstances contribute to the complexity of the disease and make it equally difficult to conduct clinical trials and basic research.
The understanding of sarcoma and the treatment of sarcoma patients has improved significantly in recent decades, with the integration of multidisciplinary treatment and molecular diagnostics being the most important achievements in clinical practice. However, physicians caring for sarcoma patients are still confronted with a variety of clinical scenarios and regularly have to make decisions based on sparse or even missing data. Even if the experience available at large sarcoma centers in the treatment of sarcoma patients can partially compensate for the lack of objective evidence, this experience also has its limits, especially in extremely rare diseases.
Sarcomas are rare mesenchymal tumors that can arise from connective and supporting tissue, muscle, bone and cartilage and can occur anywhere in the body. They are characterized by a great histological diversity and can have a very variable clinical course. While some tumor forms can grow aggressively and form metastases, others show a less severe development. Although certain sarcomas occur more frequently at a young age, patients of all ages are affected. All these circumstances contribute to the complexity of the disease and make it equally difficult to conduct clinical trials and basic research.
The understanding of sarcoma and the treatment of sarcoma patients has improved significantly in recent decades, with the integration of multidisciplinary treatment and molecular diagnostics being the most important achievements in clinical practice. However, physicians caring for sarcoma patients are still confronted with a variety of clinical scenarios and regularly have to make decisions based on sparse or even missing data. Even if the experience available at large sarcoma centers in the treatment of sarcoma patients can partially compensate for the lack of objective evidence, this experience also has its limits, especially in extremely rare diseases.
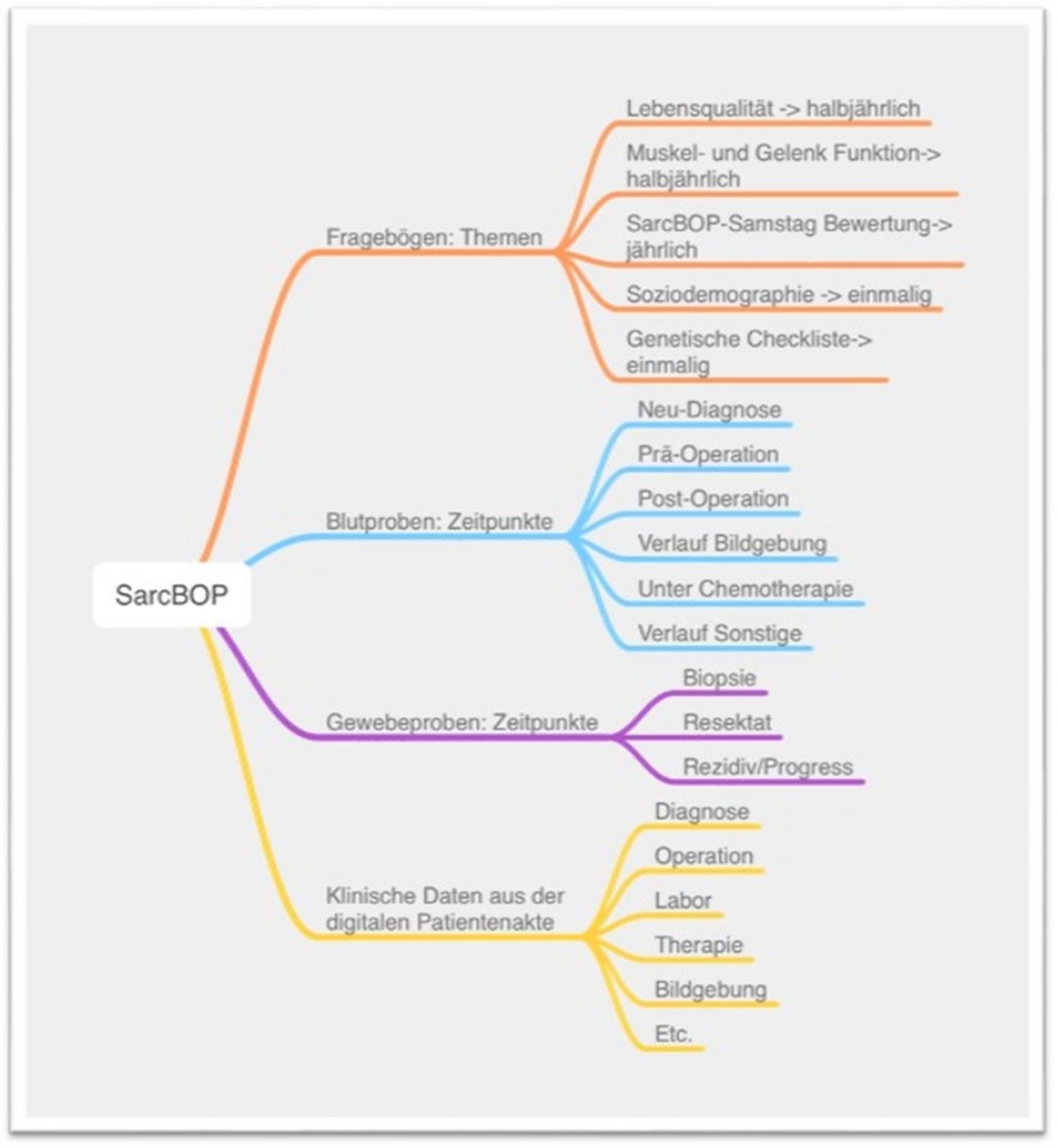
The aim of SarcBOP is to create a comprehensive database that collects, integrates and analyzes all important demographic, molecular, clinical, pathological and radiological treatment information on sarcomas. Additionally, patient questionnaires (PROs) are sent out every six months to assess and document the subjectively perceived state of health of the participants. The patient questionnaires (PROs) are either paper-based or electronic (see -> Electronic data collection with MyEDC).
The questionnaires include:
- QLQ-C30 - Quality of Life Questionnaire
- FACT-Cog - Functional Assessment
- PHQ-4 - Measurement of depression and anxiety
- PSQI - Pittsburgh Sleep Quality Questionnaire
- QLQ-FA12 - Assessment of physical, cognitive and emotional aspects of cancer-related fatigue
- Socio-demographic data
According to the affected body region:
- DASH - Impairment of the arm and shoulder
- EFAS - European Foot and Ankle Society
- OKS - Oxford Knee Score Function and Pain Questionnaire after knee joint replacement surgery
- MSTS - Musculoskeletal Tumor Society Score
- ODI - Oswestry Disability Questionnaire for back pain after spinal surgery
- OHS - Oxford Hip Score Function and Pain Questionnaire after Hip Replacement Surgery
As part of the PROSa+ research project (ethics vote: SR+BO-EK-452112024), funded by the German Cancer Aid as part of the priority program “Long-term Cancer Survivors - Data Collection and Data Analysis”, additional questionnaires can be issued once to SarcBOP patients who meet the following criteria after consenting to SarcBOP:
„Adult sarcoma survivors without active disease or living with a chronic condition ≥ 5 years after diagnosis.“
The additional, unique questionnaires within PROSa+ include:
- EORTC QLQ-SURV100 - Survivorship (quality of survival)
- GSLTPAQ - The Godin-Shephard Leisure-Time Physical Activity Questionnaire
- AUDIT-C - Alcohol Abuse Identification Test
- EORTC PATSAT-C33 - Satisfaction with cancer treatment
- SSUK-8 -Social support during illness
- ASKU - General self-efficacy
- Questions on tobacco use
- Assessment of open needs - Survey of open needs
The data collected in PROSA+ provides information about important influences of the disease on the everyday life of those affected and allows conclusions to be drawn about the physical and mental condition of patients before, during and after treatment.
In order to promote synergies between research consortia (e.g. SarcBOP and PROSa+), we make patient questionnaires (PROS) from SarcBOP available to PROSa+ for scientific evaluation, provided that patients in SarcBOP have explicitly agreed to the use of their data beyond SarcBOP.
In addition, tissue and blood samples are continuously collected in SarcBOP and stored in the biobank; the benefits of this systematic storage of tissue and blood samples are manifold:
- frozen tissue (“fresh frozen”), can be used for comprehensive molecular analyses such as whole genome and RNA sequencing as well as protein and DNA methylation analyses. Immediate storage of samples in a frozen state preserves their biological properties and molecular structures, enabling precise analyses and allowing samples to be removed at any time for various analyses if required.
- vital tissue (living cells and intact tissue structures), which is crucial for a deeper understanding of biological processes and can be used above all for individual drug testing.
- Blood samples (“liquid biopsies”) are a method that can be used for the non-invasive examination of tumors. They make it possible to extract genetic and molecular data from blood and can support the early detection of tumors as well as tumor monitoring during the course of the disease. In addition, they offer the possibility of detailed genetic analyses for targeted therapy adaptation.
SarcBOP is closely linked to the clinical activities of the Sarcoma Center Heidelberg (one of the largest centers for soft tissue and bone sarcomas in Germany), the molecular diagnostics program (DKFZ/NCT/DKTK MASTER program, NCT05852522) and our broad study program (NCT04758325, NCT06456359, NCT04625907).
SarcBOP was launched on June 19, 2019 with a positive ethics vote from the Heidelberg Ethics Committee in order to promote a translational and interdisciplinary approach to sarcoma research. Just one month later, on July 23, 2019, the first informed consent form was signed by a participant at the NCT and shortly afterwards, on August 9, 2019, the first quality of life questionnaire was completed. The first blood sample collection as part of SarcBOP followed in February 2020. Thanks to the continuous commitment and dedication of the clinicians and researchers involved, SarcBOP was gradually developed further and the integration of the Orthopaedic Clinic in Schlierbach in January 2021 further strengthened interdisciplinary collaboration.
In August 2021, additional patient questionnaires (PROS) on muscle and joint function were submitted to the Heidelberg Ethics Committee.
This enables the recording of functional limitations, the assessment of treatment success, comparability between centers, quality assurance and the further development of orthopaedic oncological care.
A key event took place with the first removal of a tissue sample as part of HEROES-AYA on November 24, 2022. SarcBOP is thus making an important contribution to the development of targeted therapies.
In June 2023, there was a new evaluation by the Heidelberg Ethics Committee. The age of the inclusion criteria was reduced from 18 to 12 years. In addition, a checklist for the indication of genetic counseling for sarcoma was included and implemented in SarcBOP.
Since the 1st quarter of 2025, patients of all age groups may be included in SarcBOP after re-evaluation by the Heidelberg Ethics Committee; an important step for pediatric oncology, especially in the field of drug testing with living tumor tissue. In addition, patients can now choose between paper-based or electronic patient questionnaires (PROS).
SarcBOP is a central component of sarcoma research and the HEROES-AYA research consortium as part of the National Decade Against Cancer.
SarcBOP also plays an important role in the certification of the Sarcoma Center Heidelberg.
To date, more than 2150 patients have been enrolled in SarcBOP, more than 2280 blood samples and more than 1275 tissue samples have been collected, and approximately 6630 PROs have been completed by participants (Figures from 25.06.2025).
Regardless of whether you are a patient, doctor or researcher - if you are interested in working with us to advance the research and treatment of sarcoma patients, we look forward to hearing from you at SarcBOP(at)nct-heidelberg.de.
News
Here you will find news about past events and milestones in the project. Take a look back at important moments!
1st SarcBOP Saturday 2022: Success at the first attempt (only in German)
2nd SarcBOP Saturday 2023: Exciting programme and fruitful discussions
3rd SarcBOP-Saturday 2024: Research, innovation and patient exchange (only in German)
Online symposium of the German Sarcoma Foundation
July 15, 2025 - PAMSARC: Therapy study on desmoplastic small round cell tumors (DSRCT) and synovial sarcoma (SySa)
Speaker: Prof. Dr. Richard Schlenk, Heidelberg
4th SarcBOP Saturday 2025: Research and experience in dialogue