NCT Heidelberg Trial Center
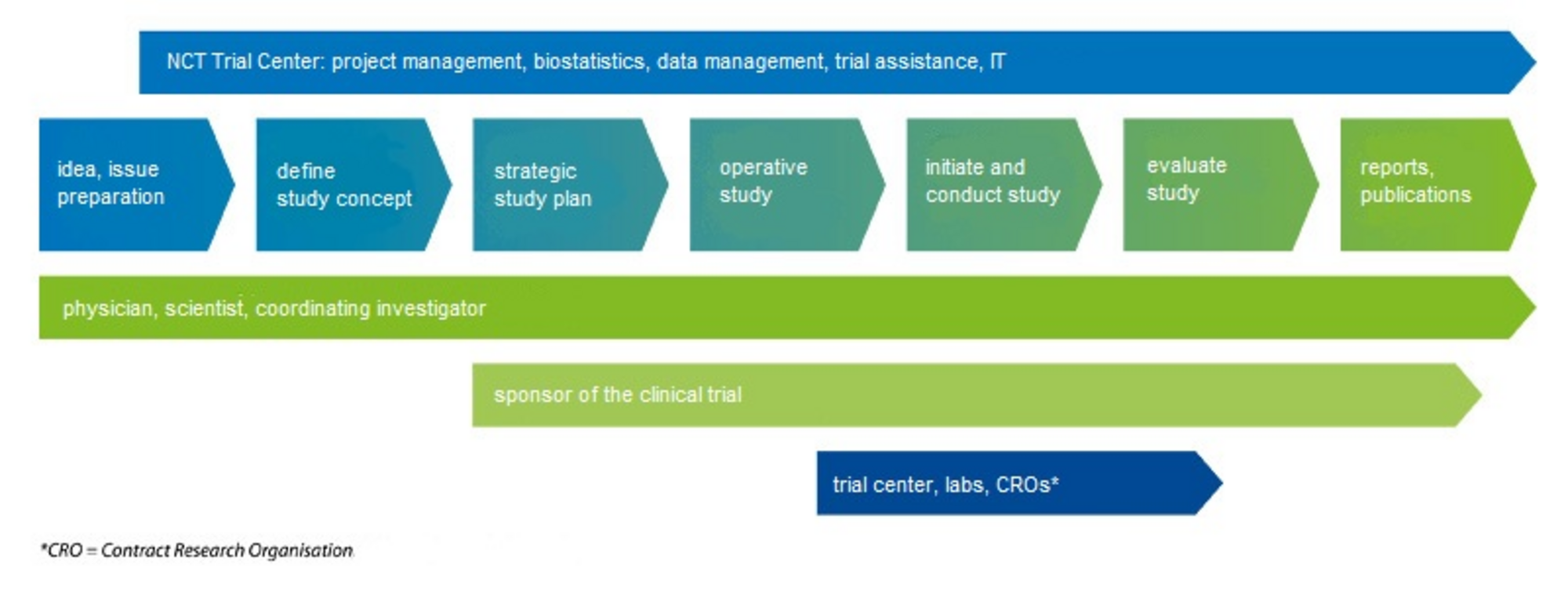
The NCT Heidelberg Trial Center is committed to support innovative academic clinical research in oncology with responsibility, expertise and dedication from competent planning over implementation to analysis and publication. Our main focus are clinical drug trials however registry studies and non-drug studies are supported as well.
We predominantly provide services for studies initiated by investigators of the partners of NCT Heidelberg - German Cancer Research Center Heidelberg and the Heidelberg University Hospital. We take over tasks of the trial sponsor and provide a wide range of services throughout all phases of clinical trials, starting with conceptual planning, application for funding, risk based quality management, preparation of trial documents and processes, over to handling of regulatory processes, implementation and conduct of clinical trials all the way to publication. Qualified, experienced and continuously trained personnel provide services in the fields of clinical project management, biostatistics, data management, quality management, study assistance and information technology.
Services
Mission: The NCT Heidelberg Trial Center ensures that clinical trials are planned, conducted, analyzed, and reported according to international standards and applicable laws. With our broad expertise we support clinical trials in all steps from planning through conduct until publication of the results. For innovative designs we develop the optimal strategies.
Data Mining and exploitation: We support scientifically driven explorative re-use of trial data to generate new hypnoses. Therefore, we develop und utilize quality-controlled processes and databases within an efficient infrastructure.
Local networking: The NCT Heidelberg Trial Center operates in close cooperation with research institutions on the campus, not only but in particular with the Coordination Center for Clinical Trials (KKS) of the Heidelberg University Hospital, the Institute of Medical Biometry and Informatics (IMBI) of the Heidelberg University as well as the Biostatistics Division of the German Cancer Research Center (DKFZ).
International networking: To facilitate the ambitious aims of NCT clinical research we network with academic research institutions locally, nationally and throughout Europe; e.g. the Cancer Core Europe consortium. We are focused on enabling academically motivated oncological studies with particular clinical significance, novel scientific issues, interdisciplinary character and new innovative drug substances and treatments.
Service request: If you are planning a clinical trial, please don´t hesitate to approach us at a very early stage and seek for our support. We are lucky to support you with trial design, feasibility considerations, budget application, and regulatory consultation. Then, please, provide us with the following Letter of Intent filled-out with your trial information as far as already known and send it at studienzentrale(at)nct-heidelberg.de.

Team and areas of operation
Our team consists of more than 40 persons:
Project Management designs and coordinates study-related processes and parties involved, timelines, and responsibilities, starting from study concept up to the final report and publication. It ensures that sponsor tasks and study topics are soundly planned and optimized according to resources, budget, contracts, and timelines, and comply with the relevant regulatory standards. During the initial planning phase Project Management
- provides regulatory advice and budgetary planning,
- supports feasibility analysis of study concepts,
- assists with funding applications, and
- drafting and negotiation of contracts.
Comprehensive quality-assured templates are available for preparing the necessary study documents during trial set-up, application with ethics committee and regulatory authorities, and trial conduct. The NCT Heidelberg Trial Center actively assists in
- preparation of study documents and
- applications and notifications and regulatory processes.
During study conduct, the Project Management serves as the central point of contact for trial sites, involved parties, and clinical monitors. It
- is contact point for all emerging issues and problems
- monitors progress, budget and timeline
- coordinates all involved partners
- monitors adherence to procedures and regulations
- performs reporting activities
- coordinates study logistics
- performs quality controls and oversees the process of risk based quality management (RBQM) and risk based monitoring
After the clinical phase of a trial is completed, the Project Management
- coordinates close-out of trial sites
- prepares the final study reports together with Principle Investigator, Biostatistics and Data Management
- ensures a timely publication
- and finally assures proper archiving of trial documentation.
Biostatistics provides scientific assistance in planning and design of clinical trials. It is responsible for statistical guidance, biometric appraisal and the statistical analysis of clinical research data obtained during the trial.
Its functions include:
- conception of clinical studies in close cooperation with internal and external partners,
- guidance in study design, target population and criteria for trial objectives,
- statistical methodology and calculation of the required sample size,
- statistical (interim) analyses, reporting, and statistical interpretation of the results.
During the preparation phase of a study, Biostatistics is responsible for development of the statistical parts of the trial protocol, the randomization plan and the statistical analysis plan. During conduct of the study, it carries out interim statistical analyses and reports. At the end of the study it is responsible for statistical analysis, the preparation of study reports and the statistical interpretation of results. It essentially supports in preparing scientific publication of trial results.
Data Management ensures the integrity, availability and validity of study data from collection to statistical analysis by making use of organizational, methodological, conceptual and technical measures. It is involved in the development of the trial protocol and study set-up. It develops Case Report Forms using modern technologies to support collection of high-quality and reliable data.
In the course of the study, the main tasks include:
- monitoring of the entire data flow including centralized monitoring supporting RBQM,
- compiling and implementation of trial-specific measures for quality control of data, and
- programming, processing and evaluating study data for reports, presentations, and study meetings during the trial as well as for the final study reports and scientific publications.
Quality assurance, documentation, and validation measures are a central aspect of all relevant process steps at data management.
Quality Management is an essential element of Good Clinical Practice (GCP) and therefore a central element of the NCT Trial Centers work. It comprises a broad spectrum of coordinated and standardized processes that are summarized in standard operating procedures (SOPs). These SOPs provide a broad spectrum of working documents, templates and checklists for planning and conducting clinical studies and analyzing study data. Risk Based Quality Management according to the current version of the ICH-GCP guidelines is supported during the whole trial duration. Auditing and assistance during inspections is an integrated part of our consultancy.
Overall, the personnel of the NCT trial center is selected carefully, broadly trained and experienced for its wide spectrum of tasks representing a high level of qualification.
Study Nurses and Clinical Trial Assistants support the clinical conduct and project management of studies. While CTAs deliver cross-functional organizational, administrative and coordinating support within the whole study process, Study Nurses provide and coordinate clinical care, assure participant safety, maintain the integrity of protocol implementation, accuracy of data collection and recording, and appropriate follow up.
Medical Information Scientists support the conduct of clinical trials esp. with data base solutions for trial management. They operate the informatics infrastructure of the NCT Trial Center and provide solutions for pseudonymisation of participant´s personal data, databases for trial management and data migration interfaces. Medical Informatics Scientists work in close cooperation with data management to guarantee a permanent availability of approaches for paperless electronic data capture and processing Furthermore, they play a responsible role in data protection measures.
FAQ
No! Please refer to the references below or contact the relevant Outpatient Clinics.
You will find on overview here:
You can find information on participating in Clinical Studies here:
You may have a look at the open positions
We encourage to send us an unsolicited application. Adress für application studienzentrale(at)nct-heidelberg.de