Head: Prof. Dr. med. Dr. rer. nat. Ungerechts
Virotherapy - Developing a unique type of cancer immunotherapy
Clinical observations of cancer remissions after viral infections laid the foundation for the field of virotherapy. Certain viruses are being explored or genetically engineered for selective replication in cancer cells, leading to tumor cell lysis. In recent years, it has become increasingly appreciated that these so-called oncolytic viruses act as a cancer immuno- (viro-) therapy via tumor vaccination effects in particular. In 2015, a first oncolytic virus was approved for the treatment of advanced melanoma in the US and Europe. Several other oncolytic viruses are currently being developed.
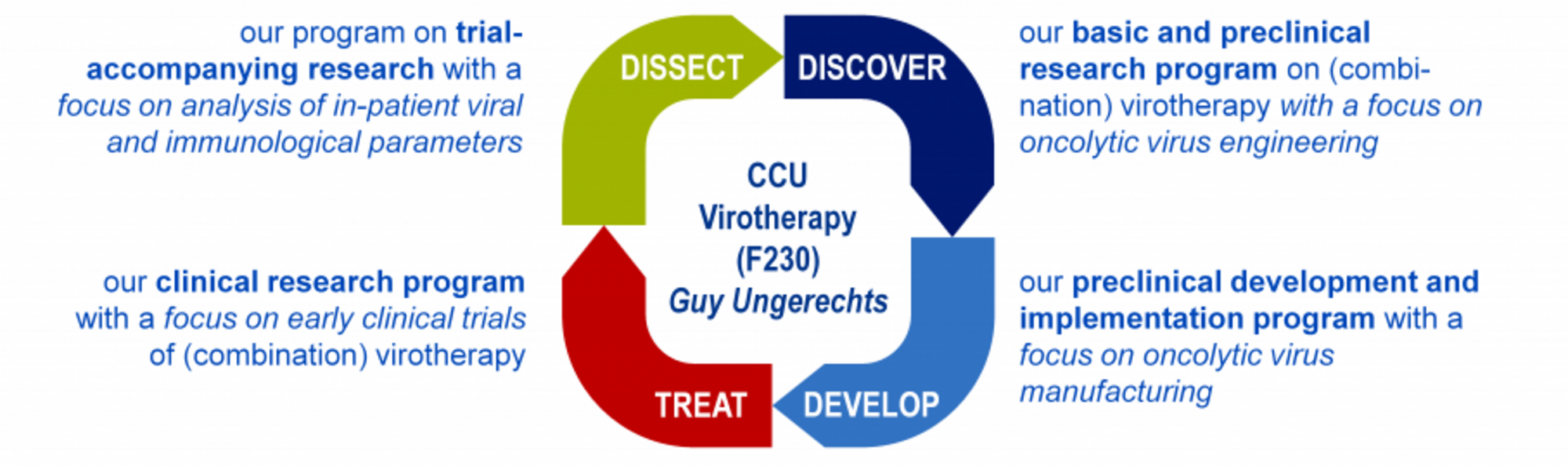
The CCU Virotherapy research agenda
We explore new strategies for engineering effective oncolytic viruses and combination therapies, establish processes for manufacturing oncolytic virus formulations, perform early and late phase clinical virotherapy studies and decipher mechanisms of oncolytic immunotherapy in preclinical models and patients.
Engineering oncolytic agents for maximum anti-tumor efficacy and safety
Our preclinical research program focuses on a measles vaccine virus platform with more recent projects exploring oncolytic adenoviruses and parvoviruses. Using molecular cloning, we engineer oncolytic viruses and explore combination regimens for specific medicinal purposes (see “discovery projects”). For instance, we have designed viruses to
- direct viral infection to tumors by selective tumor cell entry or post-entry replication control (“targeting”)
- express therapeutic proteins to implement increased potency (“arming”). Payloads include cytokines, immune checkpoint inhibitors and bispecific antibodies for alerting the patient’s immune system to the tumor (“immuno-virotherapy”)
- establish effective combination treatments (e.g. “radio-therapy”)
Deciphering mechanisms of immuno-virotherapy
We develop and apply preclinical and patient-derived tumor models and systems immunodiagnostic tools to decipher factors both within tumor cells and the tumor microenvironment that determine oncolytic potency and anti-tumor immune activation. Our research aims at rational strategies for improving oncolytic viruses and identifying potential biomarkers of immuno-virotherapy for clinical translation (see “discovery projects” and “dissect projects”).
Advancing oncolytic viruses into clinical application
With our research, we constantly aim to identify the most effective immuno-virotherapies for clinical application. Based on our preclinical findings, we are preparing a phase I/II clinical trial with an oncolytic measles virus for immuno-virotherapy of advanced gastrointestinal cancers. To this end, we currently establish GMP-compliant virus manufacturing processes (see “develop projects”). Further completed, ongoing and upcoming trials (phases I - III) explored or explore, e.g. oncolytic parvoviruses, herpes and vaccinia viruses (“treat projects”). Importantly, the trials we initiate are accompanied by translational research programs to pinpoint mechanisms of action and identify predictive biomarker signatures of successful immuno-virotherapy (see “dissect projects”).
More information on our research activities can be found here:
- Walle T, Bajaj S, Kraske JA, Rösner T, Cussigh CS, Kälber KA, Müller LJ, Boyoung Strobel S, Burghaus J, Kallenberger S, Stein-Thöringer C, Jenzer M, Schubert A, Kahle S, Williams A, Hoyler B, Zielske L, Skatula R, Sawall S, Leber MF, Kunes RZ, Krisam J, Fremd C, Schneeweiss A, Krauss J, Apostolidis L, Berger AK, Haag GM, Zschäbitz S, Halama N, Springfeld C, Kirsten R, Hassel JC, Jäger D, NCT ANTICIPATE Investigators & Ungerechts G. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nature Cancer, 2022
- Hajda J, Leuchs B, Angelova AL, Frehtman V, Rommelaere J, Mertens M, Pilz M, Kieser M, Krebs O, Dahm M, Huber B, Engeland CE, Mavratzas A, Hohmann N, Schreiber J, Jäger D, Halama N, Sedlaczek O, Gaida MM, Daniel V, Springfeld C, Ungerechts G. Phase 2 Trial of Oncolytic H-1 Parvovirus Therapy Shows Safety and Signs of Immune System Activation in Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Clin Cancer Res., 2021
- Speck T, Heidbuechel JPW, Veinalde R, Jaeger D, von Kalle C, Ball CR, Ungerechts G, Engeland CE. Targeted BiTE expression by an oncolytic vector augments therapeutic efficacy against solid tumors. Clinical Cancer Research, 2018.
- Veinalde R, Grossardt C, Hartmann L, Bourgeois-Daigneault MC, Bell JC, Jäger D, von Kalle C, Ungerechts G, Engeland CE. Oncolytic measles virus encoding interleukin-12 mediates potent antitumor effects through T cell activation. OncoImmunology, 2017.
Funding
- Deutsche Krebshilfe (PI: G. Ungerechts)
- HIPO (Heidelberg Center for Personalized Oncology) (PIs: G. Ungerechts and C. E. Engeland)
- Else Kröner-Fresenius Stiftung (PI: C. E. Engeland)
- Deutsche Forschungsgemeinschaft (PI: C. E. Engeland)
- Wilhelm Sander-Stiftung (PI: C. E. Engeland)
- NCT Elevator Pitch
- Alois Hirdt-Erben und Wieland -Stiftung Heidelberg
- Stiftung für Krebs-und Scharlachforschung
- Else Kröner Memorial Stipendium (2019 - 2021)
- Mildred Scheel MD Scholarship, Deutsche Krebshilfe (S. Anker, J. Dunder)
- Charles Conrad Award 2018 (J. Heidbüchel)
- Evangelisches Studienwerk e.V. Villigst (J. Förster)
- Anita- und Friedrich-Reutner-Preis für Medizinische Forschung 2018 (C. E. Engeland)
- Heidelberg School of Oncology Stipend (R. Veinalde)
- Helmholtz International Graduate School (Fellowships to M. F. Leber, C. E. Engeland, T. Speck, J. Heidbüchel, G. Pidelaserra Martí)
- Mildred Scheel MD Scholarship (S. Anker)
- Boehringer Ingelheim Fonds Travel Grant (J. Heidbüchel)
- Melanie and Eduard zur Hausen Foundation (Fellowship to R. Veinalde)
- MD/PhD Program, Medical Faculty Heidelberg (Fellowship to C. E. Engeland)
- Physician Scientist Program, Medical Faculty Heidelberg (Fellowships to C. E. Engeland, M. F. Leber)
- Rahel Goitein-Straus Program, Medical Faculty Heidelberg (Fellowship to E. Czink)
- Heinrich Behr Foundation (Fellowships to M. Singh, M. Bärtsch, K. Kubon)
- Ontario Institute for Cancer Research (OICR) Investigator Award 2015 (G. Ungerechts)
- Terry Fox New Investigator Award 2016 (G. Ungerechts)
Prof. Dr. Dr. Guy Ungerechts
Deputy Director Medical Oncology
guy.ungerechts(at)nct-heidelberg.de
Phone: +49 6221/42-4977
Fax: +49 6221/56-7225
Natalie Jäger
natalie.jaeger(at)dkfz-heidelberg.de
Phone: +49 6221/42-4977 (ATV) / +49 6221/56-38134 (NCT)
Fax: +49 6221/42-4809
Prof. Dr. Jean Rommelaere
j.rommelaere(at)dkfz-heidelberg.de
Phone: +49 6221/42-4960
Fax: +49 6221/42-4809
Dr. med. Elena Busch
elena.busch(at)med.uni-heidelberg.de
Phone: +49 6221/56-34643
Fax: +49 6221/56-8815
Milena Barf
Biological laboratory technician
m.barf(at)dkfz.de
Phone: +49 6221/56-5454
Fax: +49 6221/56-5217
Jessica Genz
BTA
jessica.genz(at)nct-heidelberg.de
Phone 1: +49 6221/56-35715 (NCT)
Phone 2: +49 6221/42-4931 (ATV)
Fax: +49 6221/56-5217
Stefanie Sawall
BTA
stefanie.sawall(at)dkfz-heidelberg.de
Phone 1: +49 6221/56- 5454 (NCT)
Phone 2: +49 6221/42-4931 (ATV)
Fax: +49 6221/56-5217
Katia Dittus (Günther), M.Sc
Katia.dittus(at)dkfz-heidelberg.de
Phone: +49 6221/56-5454
Fax: +49 6221/56-5217
Pierre Gommeringer, M.Sc.
pierre.gommeringer(at)dkfz-heidelberg.de
Phone 1: +49 6221/56- 5454
Phone 2: +49 6221/42- 4931
Laura Zündorf
laura.zuendorf(at)dkfz-heidelberg.de
Phone: +49 6221/56-5454
Barbara Leuchs, Dipl.-Ing (FH) Biotechnology
Vector Production & Development Unit (VP&DU), DKFZ
b.leuchs(at)dkfz-heidelberg.de
Phone: +49 6221/ 42-4300
Fax: +49 6221/ 42-4301
Alumni/Graduates
Associated Scientists:
- Prof. Dr. med. Karim Plath, geb. Zaoui
Principal Investigator:
- Christine E. Engeland
PhD Graduates:
- Martin Boos
- Gemma Pidelaserra Martí
- Gayatri Kavishwar
- Judith M. Derani (Förster)
- Christian Großardt
- Mathias Leber
- Rūta Veinalde
- Tobias Speck
- Johannes Heidbüchel
MD Graduates:
- Sophie Pernickel
- Theresa E. Schäfer
- Marc-Andrea Bärtsch
- Martin Singh
- Sophie Anker
M. Sc. Graduates:
- Katja Schaudin
- Nishika Gupta
- Annemarie Stotz
- Sergei Dumpis
- Clemens Olliger
- Aurianne Pelsma
- Katia Dittus (Günther)
- Jan Dessila
- Elise Jirovec
- Maximiliane Finkbeiner
- Alessia Floerchinger
- Judith Förster
- Laura Hartmann
- Johannes Heidbüchel
- Luisa Henkel
- Lara Jeworowski
- Mathias Leber
- Nardine Soliman
B. Sc. Graduates:
- Christine Ling Li Trautmann
- Bryce Brandenstein
Trainee:
- Celine Bauer
Internships:
- Martin Uerlich
- Sunanjay Bajaj
- Joshua Hesse
- Marie Körner
- Clemens Olliger
- Sergei Dumpis
- Victoria Gilchrist
- Linda Welte
- Gizem Altun
- Silja Schlue
- Felix Schnabel
- Saruul Jargalsaikhan
- Jennifer Schieber
- Julie Haenlin
- Hannah Briesch
Alumni:
- Dr. Sascha Bossow (Research Associate, 2009 – 2014)
- Dr. Mathias Leber (Postdoc 2014 – 2018)
- Dr. Rūta Veinalde (Postdoc 2017 – 2018)
Members of the CCU Virotherapy are involved in teaching of Oncology, Virology and Immunology for students of Medicine and Biosciences at Heidelberg University
For medical students:
HEICUMED (German)
- Modul Virologie: Praktikum und POL
- Modul Innere Medizin: POL
- Modul Gastroenterologie: Vorlesung Gastroenterologische Onkologie
- OSCE Vorbereitungskurs
- Stationsunterricht
WAHLFACHTRACK Interdisziplinäre Onkologie (German)
- Virotherapie bei Krebs: Translationale Medizin – von der Virusentwicklung zur klinischen Studie (Multimodular mit Einführung, Literaturseminar, Labor und Klinik)
- Vorlesung Onkologische Studien
- Seminar "Wie gestalte und präsentiere ich ein wissenschaftliches Poster?"
Betreuung von Studierenden in der Famulatur und im Praktischen Jahr
Journal Club Infection, Inflammation and Cancer
Medical dissertations
For students of Biosciences:
BACHELOR
- Lab rotations
- Bachelor theses
MASTER
Master Molecular Biosciences/Major Cancer Biology:
- Lecture series “Focus in Biosciences – Module 2”, Lecture “Oncolytic Viruses”
- Practical Course “HP-F6: Oncolytic Viruses and Gene Therapy”
- Practical Course “HP-F12: Immunological Methods”
Master Molecular Biosciences (Majors Cancer Biology and Infectious Diseases) and Master Molecular Biotechnology:
- Lab rotations
- Master theses
Journal Club Infection, Inflammation and Cancer
PhD supervision/Bioscience dissertations within the international PhD program of the German Cancer Research Center (https://www.dkfz.de/en/phd-program)









































