Press release from Heidelberg University Hospital (UKHD)
The German-Speaking Myeloma Multicenter Group (GMMG), headquartered at the Myeloma Center of Heidelberg University Hospital, has been successfully conducting therapy studies for 28 years. It thus makes a significant contribution to bringing research results to patient care in a timely manner. The results of a phase 3 trial have recently been published in "Lancet Haematology". They show that the antibody elotuzumab offers no additional benefit over standard therapy in patients with newly diagnosed multiple myeloma who are eligible for a stem cell transplant.
To find out how patients can be helped even better than with the available standard therapy, doctors conduct clinical trials: For example, they combine existing drugs with new ones or use established therapies in different patient groups. Although therapy studies do not always produce the positive results hoped for, they are essential in order to be able to offer scientifically sound, reliably effective therapies for every patient group.
A current example is a so-called phase 3 study on the treatment of multiple myeloma, a rare, malignant disease of the blood-forming bone marrow, which doctors from Heidelberg University Hospital, Heidelberg University and the National Center for Tumor Diseases (NCT) Heidelberg, led by Professor Dr. Hartmut Goldschmidt, have now published in the journal Lancet Haematology: In the study, 564 patients with newly diagnosed multiple myeloma at 67 German clinics and practices received the antibody elotuzumab in addition to an established drug combination. The artificially produced immune protein can suppress the disease and prolong survival in patients with recurrent or refractory multiple myeloma. However, this was not the case in the participants of the current study with recurrent disease in combination with effective standard therapy.
"We have shown that in this patient group, elotuzumab in combination with a standard therapy does not provide an advantage in terms of disease-free survival or the average three-year survival rate," says Dr. Elias Mai, Associate Professor at the Heidelberg Myeloma Center and Medical Faculty at Heidelberg University. "At the same time, however, it means that the current standard therapy, which was largely developed in Heidelberg and by the GMMG study group, is already highly effective. Elotuzumab remains an integral part of the treatment of patients with recurrent or therapy-resistant multiple myeloma."
The Heidelberg Myeloma Center of the Department of Haematology, Oncology and Rheumatology of the UKHD and the NCT Heidelberg - funded for many years by the Dietmar Hopp Foundation - is one of the largest therapy centers of its kind in the world. More than 1,400 patients a year from all over Germany and abroad are examined and treated here, mainly in studies. This enables patients to benefit from new drugs or modern immunotherapeutic procedures at an early stage. The development of new drugs, including monoclonal antibodies now used in standard therapy, has significantly improved the treatment of myeloma patients over the last 20 years: survival times have roughly doubled on average and the disease can be halted for several years in the majority of patients. However, the cancer usually returns sooner or later and then often defies treatment. "Despite the great progress that has been made, there is still a need for new treatment methods. We are therefore working tirelessly to offer and establish the latest, best possible therapies for our patients," says Dr. Mai.
The established therapies include various drug combinations and, for patients in good general health, high-dose chemotherapy followed by transplantation of the patient's own blood stem cells. The participants in the current study also received this treatment.
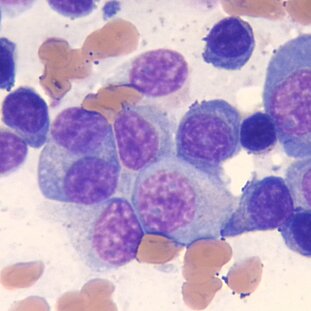
Image description
Myeloma cells in different stages of maturation (blue/purple) in a bone marrow biopsy smear.
The image is available for download here. Use of the image is free of charge, use is only permitted in connection with reporting on the topic of this press release. Use for commercial purposes is prohibited.
Literature
Mai EK, Goldschmid H, Miah K, et al. Elotuzumab, lenalidomide, bortezomib, dexamethasone, and autologous haematopoietic stem-cell transplantation for newly diagnosed multiple myeloma (GMMG-HD6): results from a randomised, phase 3 trial. Lancet Haematol. 2024;11(2):e101-e113. doi:10.1016/S2352-3026(23)00366-6
Further information on the Internet
Contact
Priv.-Doz. Dr. Elias K. Mai
Myeloma Center Heidelberg
Clinic for Hematology, Oncology, Rheumatology (Medical Clinic V) at the UKHD
National Center for Tumor Diseases (NCT) Heidelberg
Heidelberg Medical Faculty of the University of Heidelberg
E-Mail: Elias.Mai@med.uni-heidelberg.de
Phone: 06221 56-7237 (Dr. Mareike Hampel, scientific advisor)
Prof. Dr. Hartmut Goldschmidt
Head of the GMMG study group at
Heidelberg University Hospital
Heidelberg Medical Faculty of the University of Heidelberg
E-Mail: hartmut.goldschmidt@med.uni-heidelberg.de
Phone: 06221 56-8002
You can also find this press release online in our UKHD Newsroom.
Heidelberg University Hospital and Faculty of Medicine: Internationally Renowned Patient Care, Research and Teaching
Heidelberg University Hospital (Universitätsklinikum Heidelberg, UKHD) is one of the largest and most prestigious medical centers in Germany. The Medical Faculty of Heidelberg University (Medizinische Fakultät Heidelberg, MFHD) belongs to the internationally renowned biomedical research institutions in Europe. Both institutions have the common goal of developing new therapies and implementing them rapidly for patients. Heidelberg University Hospital and the Medical Faculty of Heidelberg University employs around 14.500 employees and is committed to providing trainings and qualifications. Every year, around 86,000 patients and more than 1.100.000 outpatient cases are treated in more than 50 clinical departments with almost 2.500 beds.
Together with the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) and the German Cancer Aid, the UKHD established the first National Center for Tumor Diseases (NCT) in Heidelberg. The goal is to provide care at the highest level as an oncology center of excellence and to rapidly transfer promising approaches from cancer research to the hospital. In addition, the UKHD operates in partnership with the DKFZ and the University of Heidelberg the Hopp Children’s Cancer center Heidelberg (KiTZ), a unique and nationally known therapy and research center for oncological and hematological diseases in children and adolescents.
The Heidelberg Curriculum Medicinale (HeiCuMed) is one of the top medical training programs in Germany. Currently, there are about 4.000 future physicians studying in Heidelberg.
Dr. Stefanie Seltmann
Press Officer
Head of Corporate Communications
Phone +49 6221 56-5052
Stefanie.Seltmann@med.uni-heidelberg.de
Julia Bird
Deputy Press Officer
Phone +49 6221 56-7071
Fax +49 6221 56-4544
julia.bird@med.uni-heidelberg.de