Extracellular Vesicles and Molecular Signal Transduction in Oncology
Junior Research Group - Group leader: Dr. med. Antonia Schubert
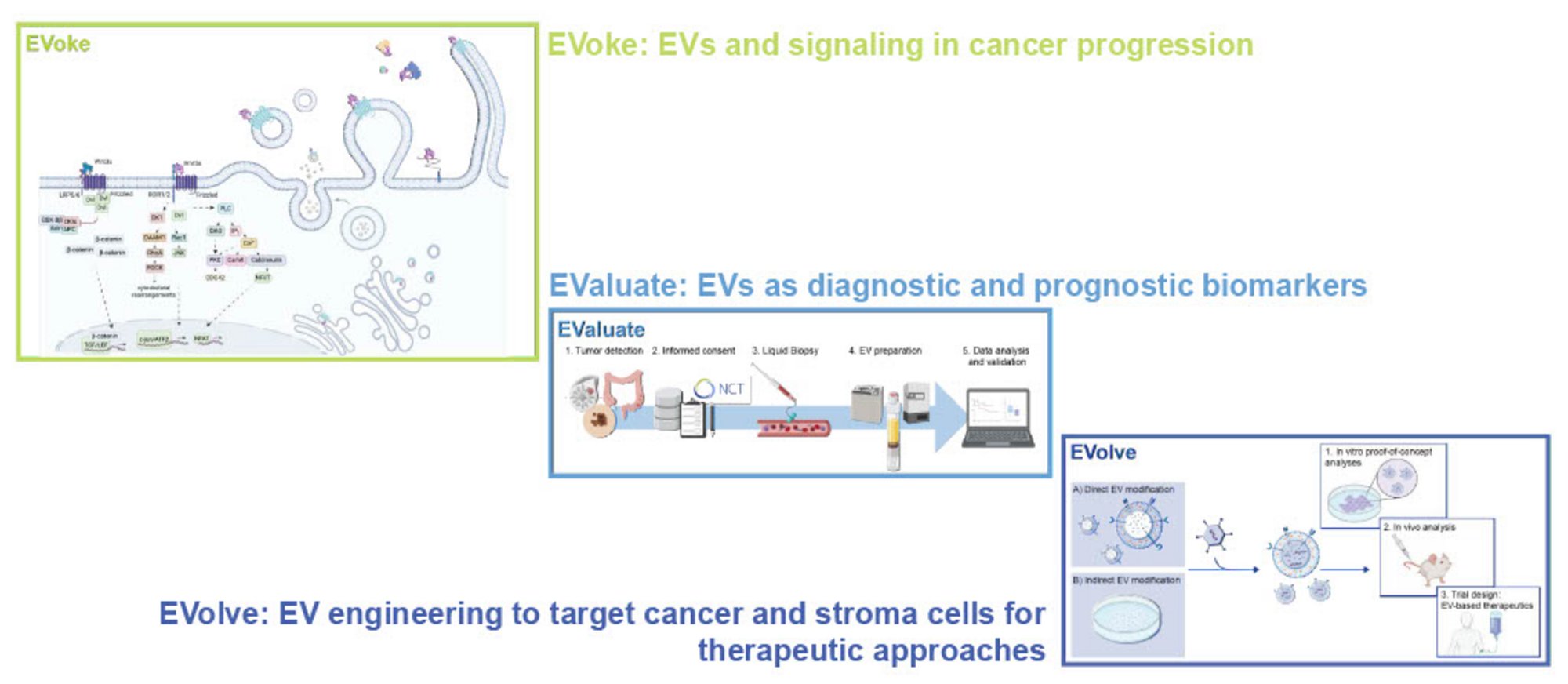
Extracellular vesicles (EVs) are key mediators of intercellular communication and play a crucial role in tumor biology –from signal transduction and tumor-stroma interactions to metastasis. Despite their high potential as biomarkers and therapeutic vehicles, their application is still limited by complex isolation procedures and an incomplete understanding of their biological functions.
Our research group bridges molecular basic research with translational oncology to systematically investigate the role of EVs in signaling pathway transduction. We address core challenges in EV research across three complementary focus areas:
We study the mechanisms of EV-mediated pathway activation and the influence of pathway mutations on EV secretion and tumor-stroma interactions.
Using a translational approach, we evaluate patient-derived EVs to evaluate their potential as diagnostic and prognostic biomarkers.
By EV engineering, we aim to explore their use as targeted delivery systems for therapeutic agents.
At the heart of our mechanistic investigations lies the Wnt signaling pathway, a major driver of tumor initiation, progression, and therapy resistance –particularly in colorectal cancer, hepatocellular carcinoma, and breast cancer. We are especially interested in how the microbiome influences Wnt-regulated signaling pathways and in the role of EVs in the complex interplay between tumor, microbiome, and therapeutic interventions.
In addition to studying extracellular signaling Wnt signals, we dissect intracellular Wnt signal transduction using genome engineering and advanced imaging techniques to functionally investigate key regulatory steps in the tumor–drug–microbiome interaction.
Our research is embedded within a vibrant network of basic science collaborations, including partners at the University of Heidelberg, University Medicine Mannheim and Göttingen, ETH Zurich, and the European Molecular Biology Laboratory (EMBL). Through our close integration with the German Cancer Research Center (DKFZ) and the National Center for Tumor Diseases (NCT) Heidelberg, we are uniquely positioned to bridge the gap between preclinical discovery and clinical application –accelerating the translation of our findings into personalized oncology.
Our overarching goal is to establish EVs as a key enabling technology in precision cancer medicine.
| 2026 | SFB 1324 Mechanisms and Functions of Wnt Signaling, Clinician Scientist-Position (A. Schubert) |
| 2025 | DFG FOR 5806 Functional Genomics and Microbiomics in Precision Medicine of Colorectal Cancer (GenoMiCC): Project Funding “Combining synthetic biology and organ-on-a-chip technology to develop molecular and microbial co-treatment strategies for increased druggability and accessibility of key targets in CRC” (Co-PI Prof. Simone Schürle, ETH Zürich) |
| 2025 | German Cancer Aid (Deutsche Krebshilfe) – Mildred Scheel PhD Fellowship (L. Schik, with M. Boutros) |
| 2023 | SFB1324 Innovation Fund: "Compositional regulation of Wnt-signaling dependent biomolecular condensates” (A. Schubert) |
| 2023 | Else Kröner-Fresenius Foundation – Project Funding: "EValuate: Characterization of extracellular vesicles as diagnostic and prognostic biomarkers” (A. Schubert) |
| 2022 | DKFZ-MOST Program (N. Winkler, with M. Boutros) |
| 2021 | DKFZ Clinician Scientist Program (A. Schubert) |
| 2018/2019 | Fellowship “Kolleg für Translationale Medizin,” University Medicine Göttingen (A. Schubert) |
| 2012 | “Gö4med” MD Fellowship, University Medicine Göttingen (A. Schubert) |
| 2025 | Poster Award, German Society for Hematology and Oncology, Köln: “High-content screens identify regulators of Dvl2 phase separation in Wnt signaling: implications for targeted cancer therapy“ (A. Schubert) |
| 2024 | DKTK Travel Grant (A. Schubert) |
| 2022 | EMBO Poster Prize, Wnt Conference Awaji, Japan: “Super-resolution microscopy localizes endogenous Dvl2 to Wnt signaling-responsive biomolecular condensates” (A. Schubert) |
| 2021 | DKTK School of Oncology Fellowship (A. Schubert) |
| 2016 | Poster Award, German Society for Hematology and Oncology, Leipzig: “DVL3 in breast cancer and tumor progression” (A. Schubert) |
Schubert A, Heigwer F, Scheeder C, Voloshanenko O, Kranz D, Ragaller F, Winkler N, Miersch T, Schmitt B, Kuhse M, Gimenes D, Ordoñez-Rueda D, Schwarz J, Stein F, Jäger D, Engel U, Boutros M. Image-based screens identify regulators of endogenous Dvl2 biomolecular condensates. BioRxiv. 2025 Jan 11:632522. doi: 10.1101/2025.01.11.632522. Preprint.
Schubert A, Mongkolsittisilp A, Kobitski A, Schulz M, Voloshanenko O, Schaffrinski M, Winkler N, Neßling M, Richter K, Kranz D, Nienhaus K, Jäger D, Trümper L, Büntzel J, Binder C, Nienhaus GU, Boutros M. WNT5a export onto extracellular vesicles studied at single-molecule and single-vesicle resolution. FEBS J. 2025. doi: 10.1111/febs.70074
Irmer B, Efing J, Reitnauer LE, Angenendt A, Heinrichs S, Schubert A, Schulz M, Binder C, Tio J, Hansen U, Geyer C, Gerwing M, Bleckmann A, Menck K. Extracellular vesicle-associated tyrosine kinase-like orphan receptors ROR1 and ROR2 promote breast cancer progression. Cell Commun Signal. 2023 Jul 10;21(1):171. doi: 10.1186/s12964-023-01186-1. PMID: 37430307; PMCID: PMC10331971.
Schubert A, Voloshanenko O, Ragaller F, Gmach P, Kranz D, Scheeder C, Miersch T, Schulz M, Trümper L, Binder C, Lampe M, Engel U, Boutros M. Superresolution microscopy localizes endogenous Dvl2 to Wnt signaling-responsive biomolecular condensates. Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2122476119. doi: 10.1073/pnas.2122476119. Epub 2022 Jul 22. PMID: 35867833; PMCID: PMC9335300.
Walle T, Bajaj S, Kraske JA, Rösner T, Cussigh CS, Kälber KA, Müller LJ, Strobel SB, Burghaus J, Kallenberger SM, Stein-Thöringer CK, Jenzer M, Schubert A, Kahle S, Williams A, Hoyler B, Zielske L, Skatula R, Sawall S, Leber MF, Kunes RZ, Krisam J, Fremd C, Schneeweiss A, Krauss J, Apostolidis L, Berger AK, Haag GM, Zschäbitz S, Halama N, Springfeld C, Kirsten R, Hassel JC, Jäger D; NCT ANTICIPATE Investigators, Ungerechts G. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat Cancer. 2022 Jun 17. doi: 10.1038/s43018-022-00398-7. Epub ahead of print. Erratum in: Nat Cancer. 2022 Jul 15: PMID: 35715501.
Schubert A, Boutros M. Extracellular vesicles and oncogenic signaling. Mol Oncol. 2021 Jan;15(1):3-26. doi: 10.1002/1878-0261.12855. Epub 2020 Dec 6. PMID: 33207034; PMCID: PMC7782092.
Bleckmann A, Conradi LC, Menck K, Schmick NA, Schubert A, Rietkötter E, Arackal J, Middel P, Schambony A, Liersch T, Homayounfar K, Beißbarth T, Klemm F, Binder C, Pukrop T. β-catenin-independent WNT signaling and Ki67 in contrast to the estrogen receptor status are prognostic and associated with poor prognosis in breast cancer liver metastases. Clin Exp Metastasis. 2016 Apr;33(4):309-23. doi: 10.1007/s10585-016-9780-3. Epub 2016 Feb 9. PMID: 26862065; PMCID: PMC4799797.