NCT Sample Processing Lab
For the analyses of whole genomes and transcriptomes, high quality analytes are required for precision and reproducibility. This applies not only to next-generation sequencing but also to many other analyses.
The NCT Sample Processing Lab (SPL) provides expertise in the controlled isolation of DNA, RNA and proteins from various sample sources including tissue biopsies, FFPE tissue blocks, blood, saliva and cells. This is followed by state-of-the-art quality control measures, such as standardized fluorometric concentration measurements and integrity check of DNA/RNA samples using automated low-volume techniques). In addition, customers can hand in analytes directly for quality control measurements.
All procedures are performed following standardized operation procedures (SOPs) and a Quality Management System in compliance with the international standard DIN EN ISO/IEC 17025. Our processes are Rili-BÄK compliant, making them suitable for the processing of samples from clinical studies. Samples are registered, tracked and stored with a unique sample identifier and every processing step is documented in the SPL database.
In addition to our participation in the Molecular Precision Oncology Program, we offer our service to the German Cancer Research Center (DKFZ) and all NCT member institution as well as members of the DKTK consortium. The SPL also supports scientific projects, clinical trails and registry studies conducted by the Medical Faculty or the Heidelberg University Hospital upon request.
Beyond our routine techniques, we are constantly developing and optimizing extraction and QC procedures to include additional starting materials and new methods. The service catalogue of our lab is presented under 'Service'. If the listed procedures do not meet your specific requirements, please feel free to contact us, and we will discuss the possibilities for custom tests and implementation.
The NCT Sample Processing Lab is an institution of the National Center for Tumor Diseases (NCT) Heidelberg under the patronage of the Medical Faculty of Heidelberg University and the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ). It is located at Technologiepark Heidelberg (Im Neuenheimer Feld 581).
The NCT Sample Processing Lab is committed to ensure the high-quality processing of samples and their derivatives for scientific research in tumour studies, as well as in the context of registry studies and clinical trials.
In close collaboration with scientists, doctors, and project coordinators, our goal is to handle each sample with the utmost care, ensuring it is prepared, analysed, and reported in accordance with our QM system, international standards and applicable regulations.
The SPL serves as crucial technology platform providing standardized workflows, highly trained staff, and a strong commitment to both internal and external process controls (e.g. reference material for extraction and quality control processes as well as proficiency testing). These factors ensure that the highest standards of quality control are maintained throughout the entire sample life cycle, including processing, analysis and storage. Wherever possible, automated processes are implemented to reduce risk of contamination and likelihood of confusion.
Since 2017, the SPL has implemented a quality management system and participate in official proficiency testing programs.
By July 2020 the SPL was accredited in accordance to the international standard DIN EN ISO/IEC 17025, which outlines the competence requirements for testing and calibration laboratories. The maintenance of compliance is monitored through regular internal audits and external assessments by the accreditation body (Deutsche Akkreditierungsstelle, DAkkS).

The current annex to our accreditation certificate, which lists the accredited parameters, is available for download here.
Our processes are Rili-BÄK compliant, making them suitable for the processing of samples from clinical studies.
In addition, we participate in he LEAF Lab Sustainability Certification Program to improve the sustainability and efficiency in our lab even more. In March 2024 we were certified with the Bronze award and in 2025 we achieved Silver.

In addition to our participation in the Molecular Precision Oncology Program, the SPL offers its service to the German Cancer Research Center (DKFZ) and all NCT member institutions as well as members of the DKTK consortium. The SPL also supports scientific projects, clinical trials and registry studies conducted by the Medical Faculty or the Heidelberg University Hospital upon request.
We have a wide experience in supporting strategic observational and interventional clinical trials, e.g., DKFZ/NCT/DKTK MASTER, CATCH, COGNITION, CAPTURE, N2M2, the NCT PMO trials, COGNITION-GUIDE, Cancer Core Europe Basket of Baskets and ESPAC6.
Alongside our primary services (please review the service catalogue below), we provide additional offerings to support various aspects of the project and ensure comprehensive solutions for our clients:
Project planning (incl. sample collection and logistics)
Support in sample logistics on campus (incl. sample transport) and if needed, support for logistics throughout Germany
Established workflows for sample transfer for follow up analysis or validation (e.g., to DKFZ Next Generation Sequencing Core Facility and Microarray Core Facility, Molecular Pathology HD)
Quality checks are harmonized with the procedures established at the DKFZ Next Generation Sequencing Core Facility and Microarray Core Facility, leading to resource-efficient sample handling.
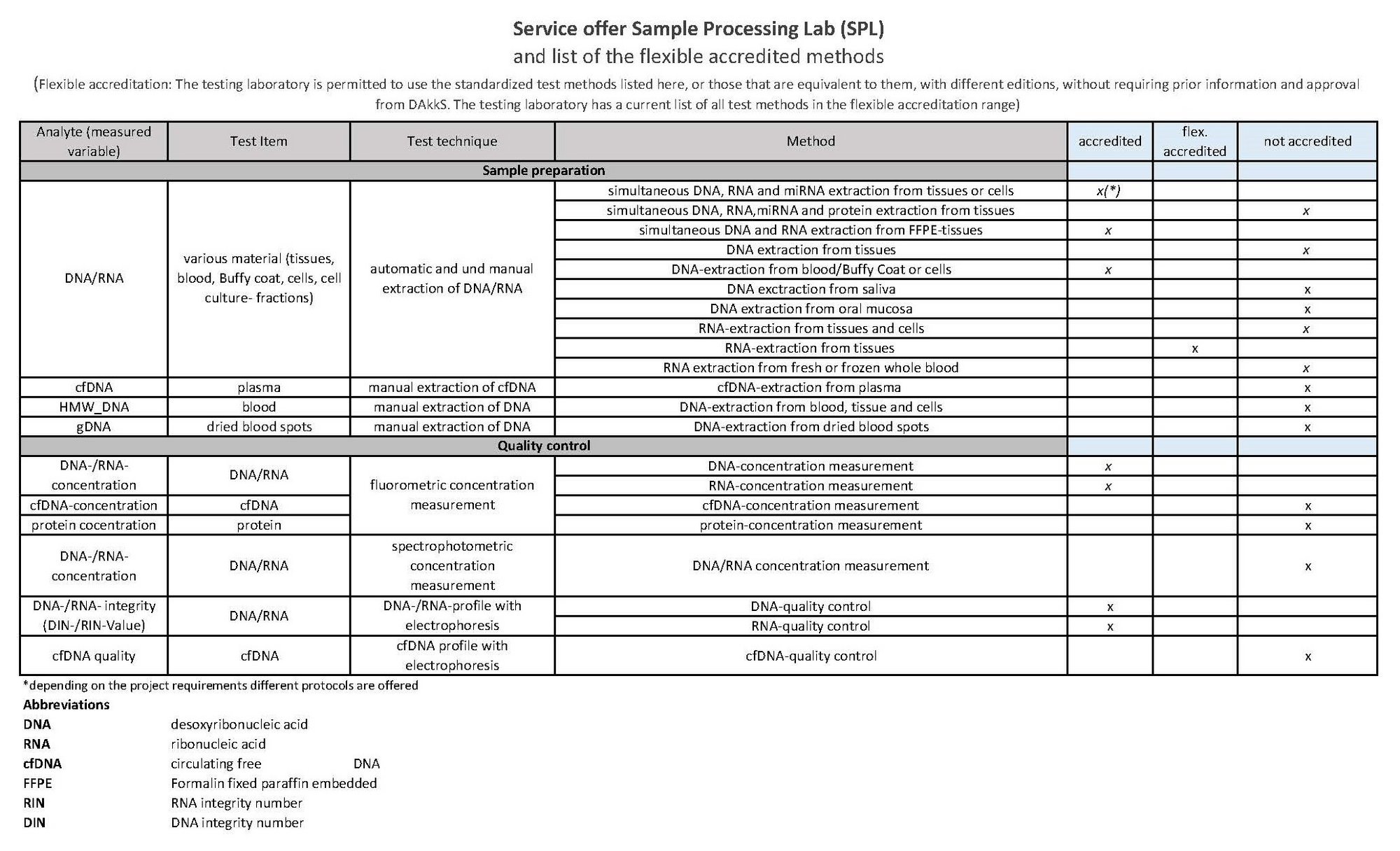
Service catalogue
(Flexible accreditation: The testing laboratory is permitted to use the standardized test methods listed here, or those that are equivalent to them, with different editions, without requiring prior information and approval from DAkkS. The testing laboratory has a current list of all test methods in the flexible accreditation range)
based on CD_B069_ADM_GE_Leistungsverzeichnis V9.0 valid from 29.10.2025
The SPL offers additional methods as R&D (Research and Development) services. These include, among others:
High molecular weight (HMW) DNA extraction from blood, tissue and cells
gDNA extraction from dried blood spots
RNA extraction from fresh and frozen whole blood
Service request
If you are planning a scientific project or clinical trial and need a central hub for sample management and processing, please feel free to contact us early in the planning phase for our support.
We are happy to assist in aligning logistics, workflows, and accompanying documentation. This enables us to better understand your requirements, not only for samples and analyses but also to assess documentation needs and estimate sample processing timelines and costs effectively.
Based on this information, a brief project proposal will be created in collaboration with the project manager. This includes determining the appropriate extraction methods, pilot tests, implementation of new methods, or optimization of existing protocols, tailored to the specific project needs.
For detailed information about our service as well as service requests, please contact Dr. Katrin Pfütze: SPL-contact(at)nct-heidelberg.de
Prices are verified by finance department and are available on request. Services must be included in the Overarching Clinical Translational Trial (OCT²) program budget. Therefore, we are keen to be involved during the project planning phase.
Please contact us for further information: SPL-contact(at)nct-heidelberg.de