On World Cancer Day: Clinical Trials Are an Obstacle Course – and Essential for Progress in Cancer Medicine
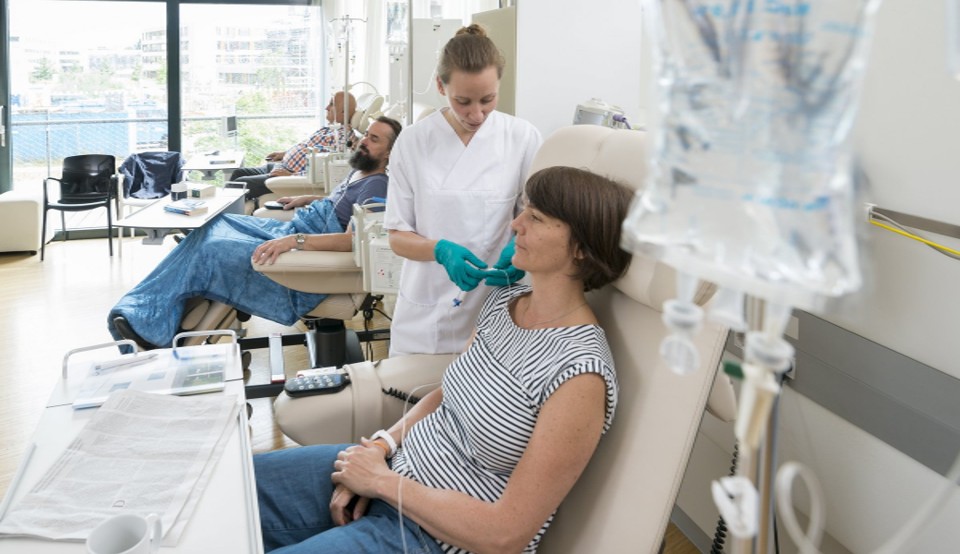
Joint press release by the German Cancer Research Center (DKFZ) and the National Center for Tumor Diseases (NCT) on World Cancer Day 2026
New cancer therapies are not developed in the laboratory alone. They must prove themselves in clinical trials before they reach patients. On World Cancer Day, the German Cancer Research Center (DKFZ), together with the National Center for Tumor Diseases (NCT), highlights the central importance of clinical trials—and the many barriers that slow their path into clinical practice.
“Clinical trials are the engine of medical progress,” says Professor Michael Baumann, Chairman of the Management Board of DKFZ and Chair of the NCT Steering Committee. “If innovations are to reach patients, studies must become faster and easier to implement.”
Professor Michael Hallek, also a member of the NCT Steering Committee, adds: “Compared with ten other Western industrialized nations, Germany ranks last in the number of clinical trials conducted per capita*. Structures such as the NCT are necessary to pool efforts nationwide and speed up implementation.”
This is exactly where the NCT comes in. Expanded in 2023, the NCT now operates six sites and provides a strong infrastructure for planning and conducting oncological clinical trials. It also improves access for people across Germany—regardless of education level, place of residence, or social background.
The expansion of the NCT is funded by the Federal Ministry of Research, Technology and Space (BMFTR) as part of the National Decade Against Cancer. DKFZ acts as the coordinating institution at all NCT sites and, together with 27 partner organizations—including 11 university hospitals—creates the structural framework for strong clinical cancer research across Germany.
A Marathon with Many Hurdles
Planning and conducting clinical trials is a lengthy process. “This is not a sprint; it’s a marathon with many hurdles,” says Julia Ritzerfeld, Head of the Clinical Trial Office at DKFZ. These hurdles include extensive bureaucratic and legal requirements as well as difficult funding conditions—all while complying with international, national, and federal regulations. This demands stamina: “Clinical trials require researchers with persistence and a high tolerance for frustration.”
Clinical Research from a Physician’s Perspective
The importance of reliable structures is also emphasized by Mirco Friedrich, Head of the Junior Research Group for Hematology and Immune Engineering at DKFZ and Principal Investigator of an NCT bridge trial (launched before the NCT expansion and now conducted across multiple sites). “Clinical trials are complex and expensive. On top of that, regulatory requirements in Germany are particularly demanding compared with the United States and neighboring European countries.” Structures such as the NCT significantly simplify the planning, coordination, and execution of clinical trials, the physician and scientist explains.
The Patient Perspective: Between Hope and Reservations
The relevance of clinical trials for those affected is underscored by Max Heller from the NCT West Patient Research Council. “Participating in a clinical trial can help patients move from a sense of powerlessness back to making their own decisions,” says Heller. At the same time, many patients have reservations about trial participation, often rooted in outdated views of cancer and medical research.
In addition, lengthy and hard-to-understand data protection information can be discouraging. Clinical trials therefore need stronger political prioritization: “They need emergency clearance—so lifesaving research does not get stuck at red lights such as data protection.”
Clinical Trials Drive Progress
On World Cancer Day, DKFZ and NCT call for continued efforts to make Germany a more attractive location for clinical trials. This requires consistent reduction of bureaucracy, further streamlining of regulatory requirements, and the development of efficient clinical infrastructures. Progress in medicine must reach the place where it matters most—patients.
* measured by number of clinical trials per capita
Source: vfa and Kearney study “Pharma-Innovationsstandort Deutschland”, 2023.
https://www.vfa.de/download/vfa-kearney-pharma-innovationsstandort-deutschland.pdf
German Cancer Research Center (DKFZ)
With more than 3,000 employees, the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) is Germany’s largest biomedical research institute. DKFZ scientists identify cancer risk factors, investigate how cancer progresses and develop new cancer prevention strategies. They are also developing new methods to diagnose tumors more precisely and treat cancer patients more successfully. The DKFZ's Cancer Information Service (KID) provides patients, interested citizens and experts with individual answers to questions relating to cancer.
To transfer promising approaches from cancer research to the clinic and thus improve the prognosis of cancer patients, the DKFZ cooperates with excellent research institutions and university hospitals throughout Germany:
- National Center for Tumor Diseases (NCT, 6 sites)
- German Cancer Consortium (DKTK, 8 sites)
- Hopp Children's Cancer Center (KiTZ) Heidelberg
- Helmholtz Institute for Translational Oncology (HI-TRON Mainz) - A Helmholtz Institute of the DKFZ
- DKFZ-Hector Cancer Institute at the University Medical Center Mannheim
- National Cancer Prevention Center (jointly with German Cancer Aid)
The DKFZ is 90 percent financed by the Federal Ministry of Research, Technology and Space and 10 percent by the state of Baden-Württemberg. The DKFZ is a member of the Helmholtz Association of German Research Centers.
The National Center for Tumor Diseases (NCT)
The NCT is a long-term cooperation between the German Cancer Research Center (DKFZ), excellent partners in university medicine and other outstanding research partners at various locations in Germany: Berlin, Dresden, Heidelberg, SouthWest (Tübingen-Stuttgart/Ulm), WERA (Würzburg with the partners Erlangen, Regensburg and Augsburg) and West (Essen/Cologne). The expansion of the NCT from the original two sites in Heidelberg and Dresden to six sites in 2023 was driven by the Federal Ministry of Education and Research as part of the National Decade Against Cancer and supported by the federal states of Baden-Württemberg, Bavaria, Berlin, North Rhine-Westphalia and Saxony.
The aim of the NCT is to translate innovations in cancer research in Germany into studies in a targeted and rapid manner in order to successfully diagnose cancer according to the latest state of research and treat it while maintaining a high quality of life. Patients are research partners at eye level.
Press Contact DKFZ
Dr. Sibylle Kohlstädt
Strategic Communication and Public Relations
German Cancer Research Center
Im Neuenheimer Feld 280
D-69120 Heidelberg
T: +49 6221 42 2843
E-Mail: S.Kohlstaedt(at)dkfz.de
E-Mail: presse(at)dkfz.de
www.dkfz.de
Press contact NCT
Janna von Greiffenstern
German Cancer Research Center
NCT Communications
Im Neuenheimer Feld 280
69120 Heidelberg
Phone +49 6221 42 2255
janna.vongreiffenstern(at)dkfz.de
www.nct.dkfz.de
Image description: Patients receive infusion therapy as part of a clinical trial in an oncology day clinic. Such studies assess efficacy, safety, and feasibility of new cancer drugs under standardized conditions.
Image source: Schwerdt / DKFZ







